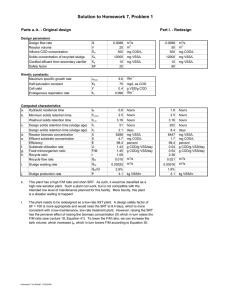
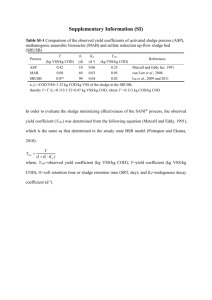
Other useful expressions:
advertisement

Other useful expressions: Qw Xr 1 mS kd VX c K s S r 1 Y su k d c X But rg = - Y rsu - kdX rg/X = = - Y rsu /X - kd where is the specific biomass growth rate (gVSS/g VSS●d) 1 therefore c Book defines Specific Substrate Utilization Rate U (g COD/g VSS●d) r Q(So S) (So S) U su X VX X Notes: Book gives F/M as QSo/VX Minimum SRT (SRTmin or min) is given by: 1 min m kd Y k kd Mixed Liquor Solids Production The total MLVSS in the aeration tank equals the biomass concentration X and the nonbiodegradable VSS (nbVSS) concentration Xi XT = X + Xi Using a mass balance, we have the following: Y(S o S) X T c (f d )( k d ) X c 1 ( k d ) c Heterotrophic Biomass (A) Where Xo,i fd cell debris (B) Nonbiodegradable VSS in influent (C) = nbVSS (nonbiodegradable VSS) concentration in influent = fraction of biomass that remains as cell debris (0.10 - 0.15 gVSS/gVSS) XTV c = total solids wasted daily (g VSS/day) = total MLVSS concentration in aeration tank (g VSS/m3) = volume of reactor (m3) Total Sludge Produced daily where X o, i c PXT,VSS XT V = PXT , VSS Daily VSS production rate: Q Y (S o S) (f d )( k d )YQ (S o S) c PX, VSS Q X o, i 1 k d c 1 (k d ) c Heterotrophic Biomass (A) cell debris (B) Nonbiodegradable VSS in influent (C) If nitrification is involved, add the following term + where NOx kd,n Q Y ( NO x ) 1 k d, n c = concentration of NH4-N in the influent flow that is nitrified = endogeneous decay for nitrifying organisms (g VSS/g VSS●d) If the reactor is a plug flow reactor: Similar analysis can be made and the final equations are as follows: cY(So S ) X (1 k dc ) _ where X = average biomass concentration in the reactor, i.e., for c/ > 5 1 c Y m (S o S) S (S o S ) (1 r )( K s ln i ) S kd r = recycle ratio Qr/Q Si = concentration of substrate after mixing with recycled sludge (mg/L) = (So+ rS)/(1+r) Oxygen Requirement - needed to (i) satisfy BOD requirements (ii) satisfy the endogeneous respiration (iii) provide adequate mixing (iv) maintain a minimum dissolved oxygen of 1 to 2 mg/L throughout the tank. Air is supplied with submerged porous diffusers or air nozzles or wastewater is agitated mechanically to promote turbulence and dissolution of air from the atmosphere (see handout of different aerators) How do we determine the oxygen requirements? Rule of Thumb Conventional systems - assume 1 lb of O2 /lb of BOD5 removed Diffused air aeration 500 - 900 ft3 air/lb BOD5 3.7 - 15 m3 air/m3 wastewater Ten States Standard 1000 ft3 air/lb BOD5 Extended Aeration 2000 ft3 air/lb BOD5 Theoretical calculations -oxygen demand is equal to the BODL (the ultimate oxygen demand) lb O2/d = Q(So - Se)8.34/f - 1.42 Px where Q So Se f 8.34 Px,bio = flow (mgd) = influent BOD5 (mg/L) = effluent BOD5 (mg/L) = factor to convert BOD5 to BODL (0.45 - 0.68) = conversion factor [(lb/mgd)(mg/L)] = mass of sludge as VSS wasted (lb/d) = QwXvr 1.42 = amount of O2 needed to convert 1 lb of cells to CO2, H2O during endogeneous decay (example, C5H7NO2 + 5O2 == > 5CO2 + 2H2O + NH3 + energy 112 160 1 1.42 Book equation kg/d = Q(So - S) - 1.42 Px,bio where So, S are expressed as biodegradable COD or bCOD If nitrification is occurring, have to account for conversion of NH 3 => NO3lb O2/d for nitrification = 4.33Q(No - N) x 8.34 No N 4.33 = influent Total Kjeldahl nitrogen, TKN = effluent TKN = conversion factor for amount of oxygen required for complete oxidation of TKN Note that the total amount of oxygen needed is usually designed with a safety factor of at least 2 times the average BOD load, to account for peak organic loading and to maintain the minimum DO level during peak loading. Wastewater Characterization (Table 8-2, Figure 8-4) Total COD (COD or tCOD) Carbonaceous materials in wastewater Biodegradable COD (bCOD) Nonbiodegradable COD (nbCOD, uCOD) Carbonaceous materials which can be degraded biologically Typically 50 – 70% Carbonaceous materials which are inert and not processed biologically Typically 30 – 50% Readily Biodegradable (soluble) rbCOD Slowly Biodegradable (particulate) sbCOD Carbonaceous materials of low molecular size, important in high rate denitrification and P removal Typically 8 – 25% Usually the main biodegradable carbonaceous fraction, require enzymatic or hydrolytic conversion, important in slow rate denitrification Typically 75 – 90% Nonbiodegradable (soluble) nbsCOD Nonbiodegradable (particulate) nbpCOD, upCOD Always present in influent, passes through plant unchanged, become effluent soluble COD (sCODe, usCODe) Inert particulate material assumed entrapped by activated sludge flocs, removed by wasting of sludge Analogous to nbVSS COD = bCOD + nbCOD bCOD consists of soluble (dissolved), colloidal and particulate biodegradable materials bCOD (1.6 to 1.7) (BOD) [note for domestic wastewater UBOD 1.5 (BOD)] (see Equation 8-1 for relationship between bCOD, UBOD, and BOD) nbCOD = sCODe + nbpCOD bCOD = rbCOD + sbCOD In addition to the above COD can be divided into soluble or dissolved COD and particulate COD. This is found by filtering the sample with a 0.45 micron filter. COD = sCOD + pCOD sCOD = rbCOD + nbsCOD + a small fraction of colloidal COD Another term that is used is biodegradable soluble COD (bsCOD) to quantify the fate of soluble biodegradable organic compounds rbCOD is determined from the oxygen uptake rate (OUR) or estimated by physical separation technique. (1) OUR – The wastewater sample and an acclimated activated sludge are mixed in a batch reactor with separate mixing and aeration. Initially with mixing but no aeration, the DO in the mixture will decline until the DO is about 3 mg/L. The OUR can be determined for the initial mixing but no aeration in terms of mg/L/h. Vigorous aeration is applied and the DO elevated to 5 to 6 mg/L. Aeration is stopped and the OUR measured. The initial OUR is used to estimated the rbCOD. rbCOD VAS Vww OA 1 YH ,COD Vww Where OA YH,COD = oxygen consumed in area A of OUR plot = synthesis yield coefficient for heterotrophic bacteria, g cell COD/g COD used = volume of activated sludge used in test, mL = volume of wastewater sample, mL VAS VWW (2) By floc/filtration method on both wastewater sample and a secondary effluent sample or a settled supernatant sample after sufficient contact and aeration of the wastewater sample with activated sludge. Zinc sulfate solution is added to a sample, pH raised for floc formation, flocs are settled, and supernatant filtered with 0.45 micron filter and filtrate analyze for COD concentration. The difference between the COD concentration between the wastewater and activated sludge treated wastewater sample is the rbCOD. Nitrogen Compounds TKN = NH4-N + Organic Nitrogen (ON) ON = bON nbON = nbsON + nbpON + nbON The nonbiodegradable particulate organic nitrogen (nbpON) can be estimated by an analysis of the influent VSS for organic nitrogen and the estimated amount of nbVSS. The fraction of nitrogen in the VSS is as follows: fN (TKN sON NH 4asN) VSS nbpON = fN (nbsVSS) Biomass solids TSS - total suspended solids VSS - volatile suspended solids MLSS - mixed liquor suspended solids (mixture of solids resulting from combining recyled sludge with influent wastewater in bioreactor) MLVSS - mixed liquor volatile suspended solids nbVSS - nonbiodegradable volatile suspended solids (derived from influent wastewater and is also produced as cell debris from endogeneous decay) iTSS inert inorganic total suspended solids (originated from influent wastewater) -