Waves & Radiation Summary Notes
advertisement

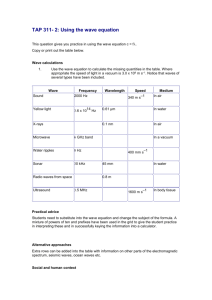
National 4/5 Physics Waves & Radiation – Section 1 - Waves Waves & Radiation Summary Notes Section 1 – Waves (National 4) Waves can transfer energy from one point to another. Longitudinal Waves A longitudinal wave vibrates along the same line as the direction of the wave energy. Sound energy is a longitudinal wave. Transverse Waves In a transverse wave the vibrations making up the wave vibrate at 90º to the direction of the wave’s energy. All members of the electromagnetic (em) spectrum are transverse waves e.g. radio, microwaves, infra-red, light, ultraviolet, x-rays, gamma rays. KD May 2012 1 National 4/5 Physics Waves & Radiation – Section 1 - Waves The Speed of Sound (National 4) The speed of sound in air is much less than the speed of light. o Speed of sound in air is approx. 340 m/s o Speed of light in air is approx. 300,000,000 m/s (3 x 10 8 m/s) An example of this is when a flash of lightning (light) is seen before the thunder (sound) is heard, although both were produced at the same time. Measuring the Speed of Sound o o o o Make a loud sound (bell) near 1st microphone. When the sound reaches 1st microphone the electronic timer starts. When the sound reaches 2nd microphone the electronic timer stops. Measure the distance (d) between the microphones. Speed of sound (m/s) = KD May 2012 distance (m) time on timer (s) 2 National 4/5 Physics Waves & Radiation – Section 1 - Waves How sound energy is transferred (National 4) Speed, Distance and Time (sound waves) speed = distance time d v t d=vxt v=d/t t=d/v KD May 2012 3 Speed (v) measured in (m/s) Distance (d) measured in (m) Time (t) measured in (s) National 4/5 Physics Waves & Radiation – Section 1 - Waves Wave Terms (National 4) Wavelength, (m) ● Distance from one point on a wave to the same point on the next wave. Amplitude, a (m) ● Size of maximum disturbance from centre (zero) position. The greater the amplitude, the more energy in the wave. Frequency, f (Hz) ● Number of waves passing a point each second. Frequency = number of waves time (seconds) Wave speed, v (m/s) ●Distance travelled by the wave in one second. Speed = distance travelled (m) time (seconds) Period, T (s) ●Time taken for one wave to pass a point Period = time number of waves. T KD May 2012 1 f or 4 f 1 T National 4/5 Physics Waves & Radiation – Section 1 - Waves The Wave Equation (National 4) Speed = frequency x wavelength V = (m/s) f (Hz) x Wave speed (m/s) Wavelength (m) v Wave frequency (Hz) (m) f Equivalence of v = f and v= d/t (National 5) Waves move a distance of one wavelength () in a time equal to one period (T). So when d = then t = T However, T = 1 f Therefore if v KD May 2012 d , v 1 t f f 5 National 4/5 Physics Waves & Radiation – Section 1 - Waves Wave Patterns (National 4) The greater the frequency of a sound wave, the higher the pitch of the sound. The greater the amplitude of a sound wave, the more energy it has, therefore, the louder it is. Electrical signal patterns in telephone wires show the same changes as those for sound signals when loudness and frequency are altered. KD May 2012 6 National 4/5 Physics Waves & Radiation – Section 1 - Waves Sound Level (National 4) The human ear can be damaged by prolonged exposure to very loud sounds. We can measure the sound intensity using a sound level meter. Sound level is measured in decibels (dB) Sound levels over 80dB are considered to be noise pollution which could cause damage to our hearing over a prolonged period, so ear defenders should be worn. The picture below gives typical sound levels in our everyday lives. The least sound intensity our ears can detect is 0 dB. KD May 2012 7 National 4/5 Physics Waves & Radiation – Section 1 - Waves Ultrasound (National 4) The frequency range for human hearing is 20 – 20,000 Hz. High frequency sound beyond this range is called Ultrasound. The pictures below show how ultrasound is used in industry, medicine and nature. o Ships can measure water depth using ultrasound o Ultrasound can be used to shatter kidney stones. o Bats move around using ultrasound KD May 2012 8 National 4/5 Physics Waves & Radiation – Section 1 - Waves Ultrasound Calculations (National 5) An ultrasound pulse is transmitted by a wave generator which acts as both a transmitter and a receiver. When calculating the distance travelled by the ultrasonic wave, we must remember that the wave is reflected off an object and travels back to the generator (receiver) e.g An ultrasonic pulse takes 0.4s to travel to the sea bed and back to the wave generator. If the speed of sound in water is 1500m/s, calculate the depth of water beneath the ship. v = 1500m/s t = 0.4s d=? d=vxt d = 1500 x 0.4 d = 600m Distance from ship to the sea bed and back up is 600m. Therefore, distance from ship to sea bed is 300m. Depth of water below ship is 300m. KD May 2012 9 National 4/5 Physics Waves & Radiation – Section 1 - Waves Doppler Effect (National 5) The Doppler Effect is a perceived change in frequency and wavelength of a wave. The effect occurs when an object generating waves moves towards and past you, as you remain stationary. The effect also occurs when you travel towards or away from a stationary source of waves. If the source of a wave gets closer to you, and you are standing still, then you hear the frequency get higher and the wavelength shorter. An example of this is when an express train rushes towards you whilst you stand still on the station platform. o As the train approaches, the perceived pitch of the sound coming from the train increases. If the source of a wave moves away from you, and you are standing still, then you hear the frequency get lower and the wavelength longer. o As the train moves away, the perceived pitch of the sound coming from the train decreases. Remember the pitch of the sound being produced by the train never changes – you just hear that it does! High Pitch KD May 2012 Low Pitch 10 National 4/5 Physics Waves & Radiation – Section 1 - Waves Diffraction (National 5) Diffraction is ‘the bending of waves around obstacles.’ All waves diffract to some extent, but longer wavelengths diffract more than shorter wavelengths. Radio waves have a longer wavelength than television waves and therefore can bend round obstacles such as hills, buildings or trees more easily. Therefore radio reception is better than television reception in such areas. (The distance between the lines represents the wavelength.) In the diagram above, you can see that the longer wavelength is much better at bending round the obstacle than the shorter wavelength. LONG WAVELENGTHS DIFFRACT BETTER THAN SHORT WAVELENGTHS. KD May 2012 11 National 4/5 Physics Waves & Radiation – Section 1 - Waves Reflection (National 5) The Law of Reflection states that : ‘The angle of incidence is equal to the angle of reflection’ Refraction (National 5) Refraction occurs when light moves from one medium to another and changes direction due to a change in its speed. KD May 2012 12 National 4/5 Physics Waves & Radiation – Section 1 - Waves Examples of Refraction (National 5) When the light ray enters a denser medium e.g. air to glass, it bends towards the normal due to its speed decreasing. When the light ray enters a less dense medium e.g. glass to air, it speeds up, and as a result, bends away from the normal. KD May 2012 13 National 4/5 Physics Waves & Radiation – Section 1 - Waves Applications of Refraction (National 5) Refraction of light through lenses can be used to correct sight defects. Short sight (Myopia) can be corrected using a concave lens. Long sight (Hypermetropia) can be corrected using a convex lens. Short Sight Long Sight Remember! With short sight, light comes to a focus in too short a time. Corrected with a concave lens. With long sight, light comes to a focus in too long a time. Corrected with a convex lens. KD May 2012 14 National 4/5 Physics Waves & Radiation – Section 1 - Waves Power of a lens (National 5) The power of a lens is a measurement of how good the lens is at changing the direction of the light ray (i.e. a measurement of refraction) The power is measured in Dioptres (D) Power = 1 ÷ focal length (m) 1 Power (D) Focal length (m) P f Thick lenses have a short focal length. Thin lenses have a long focal length. KD May 2012 15 National 4/5 Physics Waves & Radiation – Section 1 - Waves Total Internal Reflection (National 5) When light travels from a denser medium to a less dense medium e.g. glass to air, and the angle of incidence in glass gives a refracted angle of 90º in air, then the angle in of incidence in the glass is called the ‘critical angle’ Refracted Ray ic IncidentRay Ray Incident If the angle of incidence is greater than the critical angle, Total Internal Reflection occurs at the glass to air boundary. KD May 2012 16 National 4/5 Physics Waves & Radiation – Section 1 - Waves Applications of Total Internal Reflection Fibre Optics ‘Cat’s Eye’ retro-reflectors Periscopes KD May 2012 17 (National 5) National 4/5 Physics Waves & Radiation – Section 1 - Waves Section 2 – Radiation Electromagnetic Spectrum (National 4) Detectors of Radiation Radio & TV waves: aerials, radio telescopes. Microwaves: aerials, radio telescopes. Infra-red: thermometers, thermograms. Visible Light: photocells, photodiodes, the human eye. Ultra-violet: photographic film, photocells, fluorescence of certain chemicals. X-rays: photographic film, ionisation chambers, fluorescence of certain chemicals. Gamma-rays: GM tube, photographic film, gamma camera. (National 5) KD May 2012 18 All electromagnetic waves travel at the speed of light 300,000,000m/s Electromagnetic waves can travel through a vacuum. The frequency of the electromagnetic waves increases from radio waves to gamma rays. The higher the frequency, the more energy delivered by the radiation. National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 4) Radiation is all around us. Radiation can come from natural sources and artificial sources. Natural Sources Cosmic radiation (Sun, outer space) Radiation from the Earth’s crust Radiation from the human body Artificial Sources Nuclear power stations Nuclear medicine ( X-rays, radiography) Testing nuclear weapons The radiation all around us from both natural and artificial sources is known as Background Radiation Uses of nuclear radiation Medical Destroying cancerous tumours, (gamma radiation) Diagnosing problems inside the body (gamma cameras) Sterilising medical equipment Industrial Nuclear power stations Finding faults in building materials and sources of pollution/leaks Smoke alarms Radiation can damage living cells. Various precautions will reduce the harmful biological effects of radiation: o Increasing the distance between the radiation source and person. o Reducing the exposure time to radiation source. o Wearing shielding e.g. lead apron KD May 2012 19 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) Some elements in the periodic table are radioactive. This is due to the nuclei of the atoms within that element disintegrating and emitting radiation. Alpha Emitted from the nucleus. 2 protons (+ charge) 2 neutrons (0 charge) Beta Gamma An electron ( - charge) Emitted from the nucleus when a neutron changes into a proton, emitting an electron. An electromagnetic wave. Emitted from the nucleus. These are ionising radiations. Ionisation occurs when an atom loses or gains an electron. Removing and electron creates a positive ion, and adding an electron creates a negative ion. Alpha radiation is the most ionising since it is positively charged, and therefore seeks electrons. If it removes an electron a positively charged ion is left behind. KD May 2012 20 National 4/5 Physics Waves & Radiation – Section 1 - Waves Absorbers (National 5) . o Alpha can be absorbed by a few centimeters of air or a piece of paper. o Beta can be absorbed by approx 5mm of Aluminium. o Gamma can be absorbed by a few centimeters of Lead. Activity of a radioactive source o The activity of a radioactive source is a measurement of the number of nuclear disintegrations each second. o Activity is measured in Becquerel’s, (Bq) Activity A = N, number of Disintegrations (Bq) t, time (s) o The activity of a radioactive source decreases with time. KD May 2012 21 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) Half – Life When a radioactive source disintegrates, the activity (number of disintegrations per second) depends only on the number of radioactive nuclei present, so the greater the number of nuclei disintegrating each second, the greater the activity of the source. o The ‘half-life’ of a radioactive substance is the time taken for half of the radioactive nuclei to disintegrate i.e. the time taken for the activity of the radioactive source to fall to half of its original value. o We can find the half-life of a radioactive source from an Activity/time graph. o The half-life of a radioactive source is a constant value for a radioactive source. o Half-life can be measured in seconds, minutes, hours, days or years. KD May 2012 22 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) We can also find the half-life using numerical data: A radioactive isotope gives an initial count rate of 1800 Bq, after 120 minutes the activity is 225 Bq, calculate the half-life. Activity (Bq) 1800 900 450 225 Time (mins) 0 T1/2 T1/2 T1/2 3 half-lives (T1/2) = 120 minutes, therefore T1/2 = 40 minutes. Absorbed Dose (D) o The effects of absorbed radiation depend not only on the amount of radiation absorbed, but the mass of the object which absorbs it. o The term ‘absorbed dose’ is a scientific term and is defined as the energy per unit mass. It is measured in Grays (Gy). Absorbed Dose = Energy absorbed ÷ mass of absorbing material Joules (J) E Grays (Gy) KD May 2012 m D 23 Mass (kg) National 4/5 Physics Waves & Radiation – Section 1 - Waves Equivalent Dose (H) (National 5) o The biological risk caused by the absorption of radiation is represented by a quantity called the ‘Equivalent Dose’. Equivalent Dose is measured in Sieverts, (Sv) o The Equivalent Dose takes into account not only the energy of the radiation, but the type of radiation being absorbed. o Each type of radiation (,,) is given a radiation weighting factor, Wr. Radiation Radiation Weighting factor (wr) 20 Alpha 1 Beta 1 Gamma Fast neutrons 10 Equivalent Dose (H) Equivalent Dose (Sv) H Absorbed Dose (Gy) D wr Radiation Weighting Factor (No units) To calculate the Equivalent Dose when a person is exposed to more than one type of radiation, we calculate the equivalent dose for each type of radiation using the above equation and add them all together. KD May 2012 24 National 4/5 Physics Waves & Radiation – Section 1 - Waves Example (National 5) A worker is exposed to: o 15mGy of gamma radiation o 400Gy of alpha particles o 600Gy of beta particles Wr = 1 Wr = 20 Wr = 1 Calculate the total Equivalent Dose received by the worker. Gamma : H = DWr H = 15 x10-3 x 1 = 15 x10-3 Sv Total H = (15 + 8 + 0.6) x 10-3 Sv = 23.6mSv Alpha : H = DWr = 0.0236Sv H = 400 x 10-6 x 20 = 8 x 10-3 Sv Beta : H = DWr H = 600 x 10-6 x 1 H = 0.6 x 10-3 Sv KD May 2012 25 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) Fission In a fission reaction, a large unstable nucleus splits into two smaller nuclei with the release of several protons and energy. o Fission can be spontaneous, where it occurs randomly in nature, or it can be stimulated. o In a stimulated fission reaction, which occurs in nuclear reactor core, the large uranium nucleus is struck by a moderated neutron, forcing it to fission (split) into as shown in Figure 1 above. o If the mass before the fission reaction and the mass after fission are calculated, there is always some missing mass as a result of fission. o The missing mass is known as ‘lost mass’ and is changed into pure energy according to Einstein’s famous equation E = mc2 E = energy released (J) m = ‘lost mass’ (kg) c = speed of light ( 300,000,000m/s) KD May 2012 26 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) Fission o A fission reaction occurs inside a nuclear reactor. o The neutrons released go on to create more fission reactions. o This is called a Chain Reaction. The chain reaction can be stopped by absorbing the neutrons using control rods. o The energy released in a reactor’s core is used to raise steam, which turns a turbine which spins the generator rotor, producing electricity. KD May 2012 27 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation (National 5) Fusion In a fusion reaction, two light nuclei combine to form one larger nucleus and energy is given out in the process. o If the mass before the fusion and the mass after the fusion are calculated, there is always some missing mass. o The missing mass is changed into energy according to Einstein’s famous equation E = mc2 E = energy given out (J) m = ‘lost mass’ (kg) c = speed of light (300,000,000 m/s) Deuterium and Tritium are isotopes of Hydrogen. During fusion, they form a nucleus of Helium and a neutron. The two smaller nuclei need to collide at very high speeds for fusion to occur. In practice this means raising the temperature of Hydrogen gas to a staggering 100 million degrees Celsius. This happens naturally in our sun. Scientists have found this reaction very difficult to replicate on Earth due to the high temperatures needed. If we could harness the energy from fusion the world’s energy worries would be over. 10 grams of Deuterium, which can be extracted from 500 litres of water and 15g of Tritium produced from 30g of Lithium, would produce enough fuel for the lifetime electricity needs of an average person in an industrialized country. KD May 2012 28 National 4/5 Physics Waves & Radiation – Section 1 - Waves Radiation Natural occurrence By products of the reaction: Energy ratios: Conditions: Energy requirement: KD May 2012 (National 5) Nuclear Fission Nuclear Fusion Fission reaction does not normally occur in nature. Fission produces many highly radioactive particles. Fusion occurs in stars, such as the sun. Few radioactive particles are produced by fusion reaction. The energy released by fusion is three to four times greater than the energy released by fission. The energy released by fission is a million times greater than that released in thermal chemical reactions; but lower than the energy released by nuclear fusion. Fission reaction occurs inside nuclear piles within a concrete and lead reaction vessel. Takes little energy to split two atoms in a fission reaction. 29 Very high temperatures are required. Extremely high energy is required to bring two or more protons close enough that nuclear forces are overcome and the nuclei fuse together.