Muscle Contraction: Sliding Filament Theory Explained
advertisement

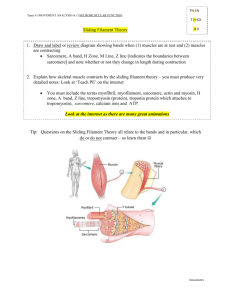
Muscle Contraction & The Sliding Filament Theory The Sliding Filament Theory explains the basis of skeletal muscle contraction and specifically deals with the movement of myosin heads along an actin fiber in the sarcomere. The first step to understanding this theory stems from an understanding of the functional parts of the muscle and its terminology. Once the components of the system are known, the mechanical process of muscle contraction follows naturally. Accordingly, this document outlines the parts of the muscle before providing a simplified explanation of the mechanism behind muscle contraction. Functional Parts of the Muscle The process behind the sliding filament theory depends heavily on the functional parts of the muscle. The muscle contains bundles of muscle fibers, with each individual muscle fiber termed a myofibril (Figure 1.1). Each myofibril represents the fusion of many cells into one large cell containing multiple nuclei. In addition, the myofibril includes the actin and myosin filaments, which together form the basis of muscle movement. The arrangement of these filaments delineates the boundaries of the muscle’s contractile unit called the sarcomere. The Sliding Filament Theory generally explains the Figure 1.1 movement of each sarcomere; accordingly, the structure of the sarcomere serves as the most important component of this mechanism. The majority of the myosin in the sarcomere Figure 1: Muscle Structure is found in the thick filaments shown in Figure 1.2, while 1.1 (above) Breakdown of skeletal muscle into its smallest parts actin myofilaments compose the thin filaments. The thin 1.2 (below) Structure of the Sarcomere filaments array themselves mainly within the I bands, while the thick filaments populate the A bands. The Z line (occasionally called “Z disk”) lays in the middle of the I band, and the M line divides the A band into two visually distinct sections. The distance from Z line to Z line determines the length of the sarcomere. Other proteins associate with the sarcomere, with titin often considered the most important of these proteins. Titin attaches the thick filaments to the Z line and extends across the sarcomere to the M line. In doing Figure 1.2 so, it acts like a “molecular ruler” and determines how far the sarcomere stretches. Another protein, nebulin, anchors the thin filaments to the Z disk. Major Steps of the Sliding Filament Theory Before any muscle contraction can take place, the muscle must receive a signal from the nervous system. Until this signal arrives, a protein complex (the troponin-tropomyosin complex) blocks the myosin head from binding to the thin filaments. Three subunits, called I, T, and C, form troponin. Troponin I prevents the actual binding of the myosin head, while Troponin T connects troponin to tropomyosin. Meanwhile, Troponin C awaits the signal from the nervous system, which releases calcium ions (Ca2+) into the sarcoplasmic reticulum surrounding the myofibril. Once released, the Ca2+ binds to Troponin C, and a conformational change in troponin allows for muscle contraction to occur. Now, the mechanism of the Sliding Filament Theory can be explained. Muscle contraction involves four major steps (shown in Figure 2): 1) ATP binds to the myosin head. The cross bridge between the two fibers breaks as the myosin head dissociates from the actin myofilament. 2) ATP is hydrolyzed. ATP breaks into ADP and Pi causing a conformational change of the myosin head into its high-energy conformation. 3) Myosin head attaches to actin myofilament. The formation of a new cross bridge leads to the release of Pi 4) Pi release triggers the “power stroke.” A conformational change in the myosin head moves the actin and myosin filaments relative to each other. 3 2 4 1 Figure 2: Sliding Filament Theory – The four major steps that result in the ultimate movement of myosin down the actin myofilament. Actin filament Step 1: ATP binds to the myosin head The thick filament contains myosin heads, which act as a molecular motor and protrude out of 1 the filament to attach to actin. At this point in the process, the myosin head initially attaches to the actin molecules to create a cross bridge between the thick and thin filaments of the sarcomere. Figure 2.1 Then, ATP binds to the myosin head. ATP (adenosine triphosphate) serves as an energy carrier in the body. When it attaches to the myosin head, the myosin head separates from the actin filament. Figure 2.1 shows the low-energy configuration of the myosin head after ATP-binding. Step 2: ATP hydrolysis In ATP hydrolysis, ATP breaks down into a lower energy state as ADP (adenosine diphosphate) and an inorganic phosphate, Pi. This transition from a higher to lower energy state releases energy, which the myosin head harvests to change conformation. The conformation change results in a “cocking” of the myosin head and prepares it for the next step. Figure 2.2 2 Step 3: Myosin head attaches to actin myofilament At this point, the myosin head enters its high-energy configuration and binds to the actin myofilament. Before this step, the ADP and Pi are still bound to the myosin head. However, upon binding to the actin filament, the Pi detaches from the myosin head. Figure 2.3 3 Step 4: The “Power Stroke” The release of Pi triggers a “power stroke” (called “working stroke” in Figure 2.4). In the power stroke, the myosin head undergoes another conformational change. With this release, the 4 myosin head pivots and moves the thin filament closer to the M line as the thick filament slides past. In doing so, the Z lines of the sarcomere move closer together, and the entire contractile unit shortens. Conclusion While these four steps may appear fairly simplistic, this cycle must repeat many times on a much larger scale than one myosin head to produce the macroscopic movement that propels animals throughout the day. In addition, 1-3% of myosin heads must attach to the thin filaments at all times to prevent backward slippage of the fibers. If all myosin heads released at once, then muscle contraction could not be sustained for longer than the milliseconds it takes for the myosin head to bind ATP, hydrolyze it, bind to the actin fiber, and trigger the power stroke. Therefore, this process occurs in staggered, multiple waves to create effective muscle contraction. Overall, the Sliding Filament Theory successfully explains the mechanism behind skeletal muscular contraction, at the microscopic level of a single sarcomere. Figure Citations Fig 1.1 & 1.2: © 1999 Addison Wesley Longman, Inc. Picture Courtesy of http://themedicalbiochemistrypage.org/muscle.html Fig. 2 (2.1, 2.2, 2.3, & 2.4): © 2001 Benjamin Cummings, an Imprint of Addison Wesley Longman, Inc. Picture Courtesy of http://legacy.owensboro.kctcs.edu/GCaplan/anat/Notes/API%20 Notes%20J%20%20Muscle%20Contraction.htm Figure 2.4 Topic Sentences The Sliding Filament Theory explains the basis of skeletal muscle contraction and specifically deals with the movement of myosin heads along an actin fiber in the sarcomere. The process behind the sliding filament theory depends heavily on the functional parts of the muscle. The Sliding Filament Theory generally explains the movement of each sarcomere; accordingly, the structure of the sarcomere acts at the most important component of this mechanism. Other proteins associate with the sarcomere, with titin often considered the most important of these proteins. Before any muscle contraction can take place, the muscle must receive a signal from the nervous system. Now, the mechanism of the Sliding Filament Theory can be explained. The thick filament contains myosin heads, which act as a molecular motor and protrude out of the filament to attach to actin. Then, ATP binds to the myosin head. In ATP hydrolysis, ATP breaks down into a lower energy state as ADP (adenosine diphosphate) and an inorganic phosphate, Pi. At this point, the myosin head enters its high-energy configuration and binds to the actin myofilament. The release of Pi triggers a “power stroke” (called “working stroke” in Figure 2.4). While these four steps may appear fairly simplistic, this cycle must repeat many times on a much larger scale than one myosin head to produce the macroscopic movement that propels animals throughout the day.