Nuclear Chemistry
advertisement

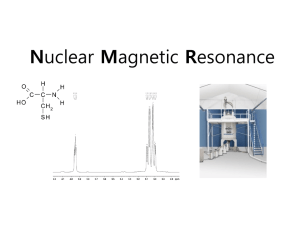
Notes 7 Spectroscopy Spectroscopy involves wavelength and frequency Certain frequencies correspond to certain types of molecular excitation Figure Experimental Particulars: ...................... Emission vs Absorption 1) Sources: Microwaves: Far IR Visible UV Far UV X-ray Gamma 2) Dispersing Elements a) Prism b) Diffraction Grating c) Fourier Transform 3) Detectors: 1) PMT 2) CCD 3) Crystal Diode Signal Modulated to help in detection Einstein said three ways for transitions to occur between states! Stimulated Absorption “B” Stimulated Emission “B” Spontaneous Emission “A” LASER - light amplification by stimulated emission of radiation Electric dipole moment operator =er permanent vs transition dipole moments A transition from one state to another occurs when the radiation field connects the two states. Spectroscopic Relations Table 10.3 As seen before the rates of stimulated and spontaneous emission are related by A=8 Spectra are seen as transition bands in a plot of intensity vs wavelength Why aren’t they just stick spectra. Why are they bands with a finite width? The band has a width due to: A. Doppler Broadening B. Lifetime Broadening .................1) collisional lifetime ................2) natural lifetime Natural linewidths increase with the magnitude of the Spontaneous Emission Coefficient What is A? Experiments measure Intensity changes Absorption Beer-Lambert Law T= A = log10(I/Io) A= cl c= l= In base e: ..................................... = ln10 .................................Naperian molar absorptivity Max value max gives indication of intensity of transition. Also talk about integrated absorption coefficient: The wavelength at which two or more components have the same extinction coefficient is called the isobestic wavelength. The absorbance is additive. It can be generalized to any number of components. The occurrence of two or more isobestics in the spectra of a series of solution of the same total concentration demonstrates the presence of two and only two components absorbing in that region. Absorption Spectra of Building Blocks (Proteins and Nucleic Acids) Most proteins and all nucleic acids are colorless in the visible region of the spectrum, but thay absorb in the near-UV region. See Fig weaker transition at 280 is a ______________________--- stronger transition at 200 nm is ____________________________________ Proteins are natural poly amino acids Nucleic acids are poly nucleotides To understand the UV absorption of proteins and/or nucleic acids, one needs to examine the various contributions to the spectra - Important Factors a. b. c. d. Amino Acid Specta Fig Polypeptide Spectra Contribution to absorption spectra from the amide linkages can be seen Fig (side chains are important) Secondary Stucture Describes which residues are in helices or other ordered conformations. The conformation of a protein is sensitively detected by UV spectroscopy. pH effects: raising the pH induces helix formation due to a reduction of the net positive charge.on the lysine side chains Fig Raising the temp converts the polypeptide to the B sheet structure. Denaturation destroys much of the secondary structure so that _______________________ ___________ occur. Nature of the Spectroscopic Changes Changes in the environment of amino acid changes the secondary structure and changes the spectroscopy. Electrical in origin. Ground and excited electronic states are sensitive to: a. b. c. d. Nucleic acids Aromatic bases attached to the ribose OR deoxyribose-phosphates all have absorption near ______________________. The free base, the nucleoside (the base attachéd to the sugar), the nucleotide (the base attached to the sugar-phosphate, and denatured poly nucleotide, all have similar absorption spectra in this region. Example: In general polynucleotides and nucleic acids absorb less per nucleotide than their constituent nucleotides. Native double stranded DNA absorbs less per nucleotide than denatured DNA stands. Decreased absorptivity is called _________________ or __________________________ Increased absorptivity is called _____________________ or _________________________ The hypochromicity of the polynucleotides or relative to the nucleotides results primarily from Fluorescence Many biological substances emit characteristic fluorescence. Chlorophyll - red fluorescence, etc. Other examples: Fluorescence labels Bioluminescence Theory of fluorescence Frank-Condon principle - the transition is vertical: Nuclei do not change position. Internal conversion Solvent Effects Excited State Properties Decay of the electronic excited state is usually 1st order. Thus there is a decay constant, a fluorescence decay time or lifetime given by: Can be other radiative processes that lead to deactivation from the excited state The quantum yield - the fraction of the absorbed photons that lead to fluorescence; it is the number of photons fluoresced divided by the number of photons absorbed. Relationship between the quantum yield and the fluorescence lifetime. Fluorescence almost always occurs from the lowest excited state of the molecule. Quenching Collision processes with specific quenching molecules leads to a change in the lifetime Stern-Volmer Relation Concentration quenching can be quite dramatic. Excitation Transfer FRET Molecular Rulers Polarization of Fluorescence Phosphorescence Single Molecule Fluorescence Optical Rotary Dispersion and Circular Dichroism Polarized Light unpolarized light plane polarized light circularly polarized light elliptically polarized light Birefringence Linear birefringence - difference in refractive index for light polarized in planes perpendicular to each other Circular birefringence - the difference in refractive index for right circularly polarized light and left circularly polarized light. Optical Rotation and Circular Dichroism Optical rotation by chiral samples results from and is a measure of their circular birefringence. Comes from the usual experimental measurement Note that phi is given per cm of pathlength in the sample. The actual rotation increases linearly with the pathlength through the sample. The rotation is measured. Circular Dichroism results from a differential absorption of left an right circularly polarized light by a sample that exhibits molecular asymmetry. The passage of plane polarized light through a circularly polarized dichroic sample produces not only a phase shift due to the circular birefringence but also a differential decrease of the amplitudes of the right and left circularly polarized components. The emerging beam is found to be elliptically polarized with ellipticity defined as See Fig and table Nucleic Acids and proteins and optical activity Circular dichroism of the synthetic polynucleotide poly (dG-dC) poly (dG-dC) Fig Vibrational Spectra Infrared Absorption Ideal Model is ___________ ______________________. The vibrational frequency is equal to: Non-ideal part occurs at high quantum numbers. It can be modeled by the Morse potential. The selection rule is based on a difference in dipole as a result of vibration. Resulting selection rules are: Pure Rotational Motion All J are forbidden unless there is a permanent dipole moment Rotational Transisitons - photons in wavelength region h = E = Epnoton = In rotation, at high quantum numbers, one can also deviate from the ideal case due to centrifugal force. This will effectively cause changes in the "Rigid" bond length at higher rotational energy. Vibration-Rotation Usually express the energy in cm-1: (wavenumbers) or 1/wavelength in cm = Need EvJ Ev,J = OR EvJ/h = e(v + ½) + Be J(J+1) - D J2(J+1)2 .............................. A1 Be = rotational constant = D = centrifugal distortion constant (corrects for ..............................................................) = So far we have made a correction for non-ideality in the Rotor (Rotation) but vibrational energy is still based on the Harmonic Oscillator One correction is to use the Morse Function for the Vibrational Kinetic Energy V(r) - V(re) = De [1 - exp(-a(r-re)]2 De = Dissociation Energy - energy required to dissociate the molecule from the state of minimum potential B Figure Corrections for the anharmonicity and for interaction between vibration and rotation give: EvJ = All three of the correction terms cause the energy levels to be lower for large values of the quantum #. At low values the terms in e, , and D may be neglected! NOW READY FOR SPECTRA Pure Rotational Spectrum E rot (in cm-1) = see fig How is this related to the bond length? How can we get the bond length from this information? Vibration Rotation Spectrum Vibrational Transitions - Infrared Region For Diatomics Selection Rules x = r-re and (x) is operator for molecular transition dipole moment. Using the Harmonic Oscillator Wavefunctions find that the selection rules are: v = 0, +/- 1 .... and .... J not = 0 v = 0 (pure rotation) v = +/- 1 (vibration - rotation) Allowed transitions between ground vibrational state (v=0) and 1st excited vibrational state. Figure Set of spectral lines result - BAND Spectral lines for which J in upper state is larger are called R branch of the band E is Higher energy, higher frequency Spectral lines for which J in upper state is smaller are If lines occurred for values of J in both states the same this would be called the Q-branch NOT OBSERVED FOR DIATOMICS - Forbidden by Selection Rules However, may be observable for polyatomics. Thus spectrum looks like: Figure Spectral peaks corresponding to v = +/-1 are called fundamentals. Vibrational selection rules are less well obeyed than rotational selection rules and often one can observe in a spectrum the transitions corresponding to v = +/- 2 ...... first overtone v = 3 ..... second overtone And in polyatomics where several vibrational modes exist can get combination bands. If we neglect the anhamonicity, the centrifugal distortion, and the coupling then the wavelength of a line of the Branches is: Light Scattering - Elastic and Inelastic light scattering Rayleigh Scattering and Raman Scattering Rayleigh Scattering Raman Scattering Stokes Anti-Stokes Resonance Raman NMR (Nuclear Magnetic Resonance Spectroscopy) NMR monitors changes in the nuclear spin state. Nuclei have intrinsic spins like electrons do. Nuclei can have integral or half integral spin states. (O, ½, 1 ….) others. Nuclei with spin ½ include H-1, C-13, N-15, and P-31. In the absence of an externally applied magnetic field, different nuclear spin states are degenerate, but in the presence of an externally applied magnetic field, the spin states hav different energy. The spin quantum numbers are related to the magnetic moment along the z-axis by The energy of a spin state in a magnetic field is: Bo is the strength of the magnetic field in Tesla directed along the z-axis. The energy difference between these two states is The energy difference is related to a frequency for the transition (spectroscopy), called the ______________________ frequency. For a proton in a field of Bo of 11.7T, the frequency is 500MHz. This frequency corresponds to radiowaves in the electromagnetic spectrum. NOTE: These are very-low energy transitions, compared with electronic or vibrational transitions. For a large number of nuclear spins, the lower and upper spin state will be nearly equally populated, since the energy splitting is much smaller than the thermal energy (kT) at room temp. Since the intensity of the absorption is related to the difference in the population of the two states, NMR is much less sensitive than IR or UVVis Absorption Spectroscopy. The energy splitting can be increase by increasing the magnetic field strength. Magnets as lar as 18.1 T are in us. Here the Larmor frequency for a proton is 800 MHz) So the vernacular is that an instrument with an 18.1 T magnet is called an 800MHz NMR. Spectrum results as a result of the nuclei absorbing energy when the radiation matches the Larmor frequency. Classical picture of NMR - Vector Model free induction decay is the return of the system to equilibrium in which a large number of spins behave in a coherent fashion. Wavefunctions, like waves, have a phase associated with them, and the wavefunctions may add together if their phases are aligned correctly. Interactions in NMR 1. Chemical Shifts - the magnetic field experienced by a nucleus is slightly different from that of the applied external field and depends on the local environment. The energy level separation between the two spin states is thus slightly changed from that causes by the applied field. As a result of this, different protons will have different resonant frequencies. The frequency of an NMR peak is usually expressed with respect to a reference frequency, using protons that resonate at a high frequency extreme of the spectrum. The resonance position is expressed in terms of a frequency difference between the reference peak and the observed peak so that it is independent of the magnitude of the applied field. This measure of resonance frequency is called the Chemical Shift. It is: Local magnetic fields that give rise to chemical shift are caused by the electrons in the molecule. Electron currents depend on the orbital configuration of the molecule. See figure 2. Spin-Spin Coupling. Scalar Coupling, or J-Coupling: The interaction of nuclei through electrons in connecting bonds depends on whether the spin state is aligned or against the magnetic field. The spin state of one nucleus affectss the spin energies of the neighboring nuclei (Spin coupling or J-coupling). The two possible orientations of a spin = ½ nucleus in a magnetic field can split the energy levels of neighboring nuclei. Thus the absorption line of a set of equivalent nuclei is split into a multiplet. The frequency of separation between the lines of the multiplet is the spin-spin splitting, J in Hertz, if the spin-spin splitting is less than one-tenth the frequency differenc due to the chemical shifts, simple first-order theory, the effects of the chemical shifts and the spin-spin splittings are additive. If the splitting between protons is comparable or larger than the difference in chemical shifts, then the spectrum depends on the ratio of J to the frequency difference. See fig The spin-spin splitting in Hertz (ulike the chemical shift) is independent of the applied magnetic field. The values of J for protons range from 0 to about 20 Hz. Proton NMR measured in a hydrocarbon or carbohydrate are not affected by the carbon or oxygen nuclei as they have no magnetic moment except a very small amont of C-13 or O-17. See Figures 3. Relaxation Mechanisms Once the energy is absorbed to change the nuclear spin state, a return of the spin population follows first-order kinetics: its relaxation time is calls the: Spin Lattice Relaxation Time, T1 Measurement of the relation rates for nuclear spin probes the local environment and dynamics of a molecule. Relaxation measurements can be used to determine whether a biological macromolecule moves together rigidly or fluidly in a solution. Another characteristic relaxation time tha is measurable in NMR is the spin-spin relaxation time, T2, which characterizes the interactions between spins on equivalent nuclei, it does not involve change of energy with the environment (the lattice). It can be measured from the width of the NMR peak. Nuclear Overhauser Effect (NOE) can be sued to determine which nuclei are near each other. Intense radiation corresponding to the transition frequency of one type of proton is applied to the sample. The NMR peak is saturated changing the spin population of those protons so there are an equal number of nuclei in the upper and lower energy levels. These nuclei interact with neighboring nuclei and change the spin population of the neighboring nuclei from their equilibrium distribution. The intensity of the NMR peak of each nearby nucleus will change Since the interaction between magnetic nuclei depends on 1/r to the 6th power, the Overhauser effect is appreciable only for very close neighbors (usually closer than 5 angstroms away). Multidimensional NMR COSY - correlated spectroscopy - two short pulses separated by a delay period T1. After the second pulse the free-induction decay is detected during the period T2 Figure NOESY - monitor through space NOE interactions between protons that are less than 5-6 angstroms apart. This is a powerful means of gaining structural information by solution NMR Fig EPR Electron paramagnetic Resonance MRI Molecules have a range of conformations produced by vibrations of all the bonds and the torsional rotations around the single bonds as discussed previously Binding of small molecules to many sites on a macromolecule and the disruption of hydrogen bonding which leads to helix-coil transitions of polynucleotides and polypeptides. Binding of Small Molecules by a Polymer Langmuir Adsorption Isotherm Statistical Thermodynamics Derivation of the Boltzmann Distribution for Individual Particles Use of the Boltzmann Distribution for Statistical Thermodynamics BUT WITH RESPECT TO SPECTROSCOPY, THE BOLTZMANN DISTRIBUTION TELLS US THE RELATIVE POPULATION OF THE STATES INVOLVED IN THE SPECTRAL TRANSITION. And since the intensity is related to the population of the initial state, then the Boltzmann Distribution is related to the intensity of a peak in a spectrum. Consider a system of N molecules Total Energy of the system is E We say that the energy is distributed over the molecules. Collisions take place - there is a ceaseless redistrubution of energy - not oly between the molecules, but also among their different modes of motion. If several energy states: translational, rotational, vibrational, electronic then the closest we can come to describing the DISTRIBUTION OF ENERGY is to STATE THE POPULATION IF EVERY ENERGY STATE or LEVEL From Quantum Mechanics we know that the energy of a molecule is not a continuous function. Remember that the energy of vibration is restricted. For our DISTRIBUTION we make the following definitions and assumptions: 1) on average there are ni molecules in a state of energy i or energy level i. 2) Population - average # may be relatively constant in a level even though collisions occur. 3) Principle of equal a priori probabilities: “All Possibilities for the distribution of Energy are equally probable ie vibrational states, rotational states, electronic states 4) Ergodic Hypothesis (Why are we interested in the distribution - defines equilibrium situation) “ The long time average of a Mechanical variable, M, of interest is equal to the ensemble average of M in the limit that N infinity, provided that the ensemble replicates the thermodynamic state. So we need a statistical treatment of an assembly of molecules to find there average arrangement, and that will represent the equilibrium thermodynamic state. Statistics shows that although there are many possible arrangements of the energy quanta within the molecules of the assembly, that only one configuration has an extremely high probability. That is the arrangement of the energy into the molecules defined by one configuration is many - many times more likely to happen than any other configuration. This particular configuration is defined by the Boltzmann Distribution: One form of the Boltzmann distribution is: The probability that a molecule is in the ith energy state (pi) is given by: pi = expi) / j exp(-j)) ............................... where = 1/kT and ni (the number of molecules in the ith level is given by: N pi ...... (where N is the total # of molecules) j exp(-j) is often denoted by Q and is called the partition function Also note that the relative probability that a molecule is in the ith level relative to the jth level is given by: ni /nj = exp (-i - j))