Chemical Ideas 12.3: Arenes
advertisement
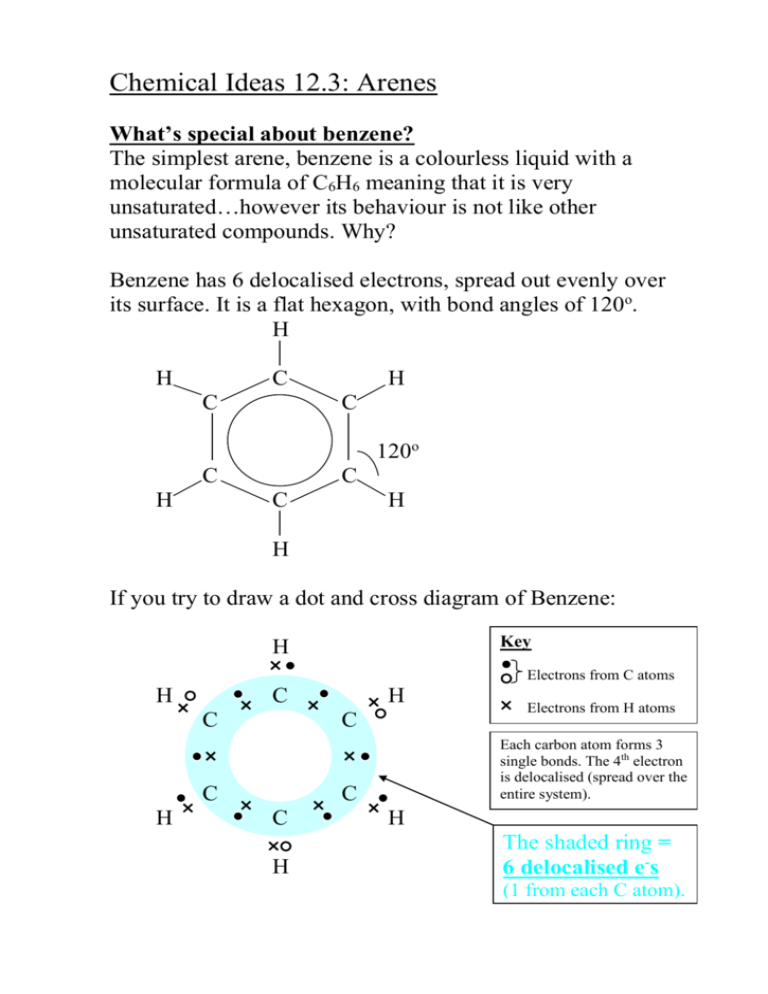
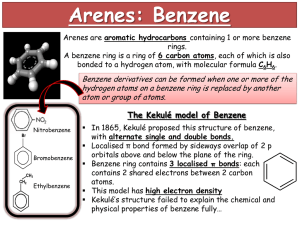
Chemical Ideas 12.3: Arenes What’s special about benzene? The simplest arene, benzene is a colourless liquid with a molecular formula of C6H6 meaning that it is very unsaturated…however its behaviour is not like other unsaturated compounds. Why? Benzene has 6 delocalised electrons, spread out evenly over its surface. It is a flat hexagon, with bond angles of 120o. H H C C H C 120o C H C C H H If you try to draw a dot and cross diagram of Benzene: Key H Electrons from C atoms H C C C C H H Each carbon atom forms 3 single bonds. The 4th electron is delocalised (spread over the entire system). C C H Electrons from H atoms H The shaded ring = 6 delocalised e-s (1 from each C atom). Electron density maps from X-ray diffraction studies give more evidence for the delocalised structure: Scale 0 0.1 nm Notice the uniform density in all the carbon-carbon bonds. August Kekulé (1865). Suggested that the delocalised structure exists in 2 forms: And called Kekulé structures. We think of benzene existing between these extremes: Stability of benzene The cyclic structure makes benzene much more stable than expected, compared to alternating single and double bonds of the kekule structures. The stable ring is preserved when benzene undergoes reactions; infact reactions in which the ring is disrupted only happen at much higher temperatures and more vigorous conditions. Consider the thermochemical data of the following reactions: + H2 H = - 208 kJ mol-1 H = - 120 kJ mol-1 + H2 The value for the Kekule structure cannot be measured, so is hypothetical… H = 3 x (- 120 kJ mol-1) + H2 = -360 kJ mol-1 Thus, when 1 mole of benzene molecules are hydrogenated, (360 kJ mol-1 - 208 kJ mol-1) = 152 kJ mol-1 less energy is given out than expected from the Kekule structures. What are arenes? ‘ar’ = aromatic (sweet-smelling/ aroma / fragrance)…not all arenes are sweet smelling – benzene smells quite strong and unpleasant! ‘-ene’ = unsaturated, like alkenes. They are sometimes called ‘aromatic hydrocarbons’ as they are hydrocarbons like benzene which contain stabilised ring structures. Hydrogen atoms are often replaced with alkyl or other functional groups: Methylbenzene 1,3-dimethylbenzene 1-ethyl-4-methylbenzene CH3 CH3 CH2-CH3 CH3 CH3 Where ONE hydrogen atom is replaced, it is referred to as a phenyl group (formula C6H5). Two important aromatic compounds whose names are based on the phenyl group are PHENOL and PHENYLAMINE. Two functional groups are numbered using the lowest numbers possible. Functional groups are named in alphabetical order. Benzenes can join in fused ring systems and share a pair of carbon atoms. Examples: Naphthalene, C10H8 anthracene, C14H10 It is important to draw the double and single bonds, to illustrate how many delocalised electrons there are. what would be the formulas of the following structures? Why are they incorrect? Compounds derived from arenes Match the following aromatic hydrocarbons to their structures: a) chlorobenzene, b) nitrobenzene, c) benzoic acid, d) benzaldehyde, e) benzyl alcohol, f) phenol, g) phenylamine. e) d) a) CH2OH CHO Cl g) NH2 b) NO2 f) OH c) COOH Do problems for 12.3 p292 questions 1- 4. Chemical Ideas 12.4: Reactions of arenes The six delocalised electrons in benzene do not belong to any carbon atom. They are spread out in a cloud above and below the plane of the benzene ring: Regions of higher electron density attract positive ions or atoms with positive charge within molecules. Benzene, (like the alkenes) reacts with electrophiles. Alkenes undergo addition reactions to form saturated molecules. H H H H C C H + Br Br H H C C Br Br H Electrophilic substitution reactions of benzene The stable ring system is kept intact, in most reactions. Bromination of benzene FeBr3 + Br Br Br + HBr Benzene reacts with the Br+ electrophile in an electrophilic substitution reaction. Bromine becomes polarised as it approaches benzene and first reacts with iron filings (used to speed up the reaction) to make iron bromide: 2Fe + 3Br2 2FeBr3 (FeBr3 catalyses the reaction by accepting a lone pair from Br2 and so making Br+ Br MORE polarised.) Br+ Br+ Br FeBr4- FeBr3 The bromine becomes so polarised that it splits into Br+ and FeBr4-. The Br+ joins to a carbon atom in the benzene ring and the H+ it replaces reacts with FeBr4- to make HBr and reform FeBr3. Br Br+ FeBr4- + H Br + FeBr3 Why doesn’t benzene undergo addition reactions? This is much more difficult, and would mean disrupting the stable ring system: Br + Br Br Br Benzene undergoes other electrophilic substitution reactions… Nitration Benzene reacts with a nitrating mixture (concentrated sulphuric acid and nitric acid) below 55oC to form nitrobenzene. NO2 + HNO3 c. HNO3 o < 55 C + H 2O The electrophile is NO2+: HNO3 + 2H2SO4 NO2 + 2H2SO4- + H3Onitrating mixture At higher temperatures, further substitution results in dinitrobenzene and tri-nitrobenzene. Sulphonation When benzene is heated under reflux for several hours with conc. sulphuric acid, benzenesulphonic acid is made: + H2SO4 SO2OH reflux + H 2O O- The electrophile is SO3: The SO3 carries a large positive charge on the sulphur atom. S+ -O O- Benzenesulphonic acid is a strong acid which forms salts in alkaline solution: SO2OH SO3- +Na + NaOH + H2O Most detergents contain salts like this with long alkyl groups attached to the benzene ring. The hydrocarbon part dissolves in fats, the ionic part dissolves in water. Chlorination Happens in a similar way to bromination… aluminium chloride is the catalyst: Overall: Cl AlCl3 + Cl Cl + H Cl First step: Cl+ Cl+ Cl AlCl4- AlCl3 reacts violently with water, so conditions must be anhydrous. AlCl3 Second step: Al Cl + AlCl4 - + H Cl + AlCl3 Friedel-Crafts reactions Aluminium chloride is used as catalyst to help polarise hydrogen-containing organic molecules and cause them to substitute into the benzene ring. Two examples are alkylation (introducing an alkyl group into the benzene ring) and acylation (introducing an acyl group). Methyl benzene is made by warming benzene with chloromethane and anhydrous aluminium chloride: CH3 + CH3 Cl + H Cl The mechanism is similar to the halogens, AlCl3 causes CH3Cl to polarise. This is an example of alkylation. Acylation (same conditions as alkylation, but treat with acyl chloride or an acid anhydride) O O C AlCl3 + CH3 C CH3 + H Cl reflux Cl (ethanoyl chloride – an acyl chloride) O + CH3 C AlCl3 reflux O C CH3 + CH3COOH O CH3 C O copy summary and learn these reactions p297 do problems for 12.4 p 297-298 questions 1-6.