2120.final.sol
advertisement
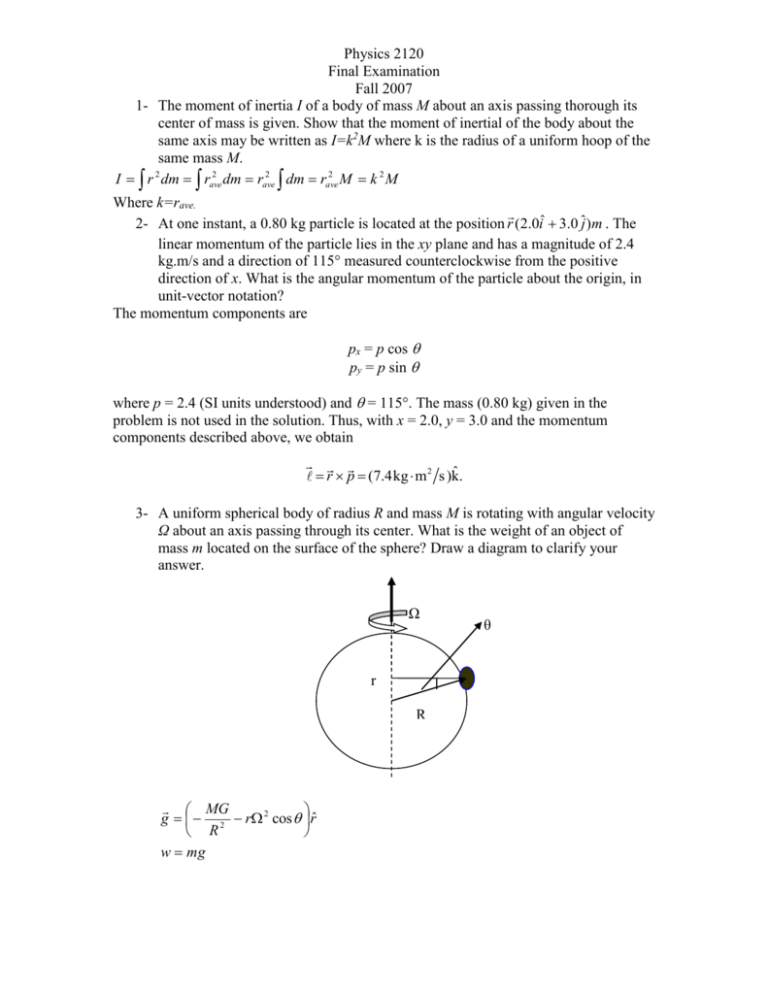
Physics 2120 Final Examination Fall 2007 1- The moment of inertia I of a body of mass M about an axis passing thorough its center of mass is given. Show that the moment of inertial of the body about the same axis may be written as I=k2M where k is the radius of a uniform hoop of the same mass M. 2 2 2 2 2 I r dm rave dm rave dm rave M k M Where k=rave. 2- At one instant, a 0.80 kg particle is located at the position r (2.0iˆ 3.0 ˆj )m . The linear momentum of the particle lies in the xy plane and has a magnitude of 2.4 kg.m/s and a direction of 115° measured counterclockwise from the positive direction of x. What is the angular momentum of the particle about the origin, in unit-vector notation? The momentum components are px = p cos py = p sin where p = 2.4 (SI units understood) and = 115°. The mass (0.80 kg) given in the problem is not used in the solution. Thus, with x = 2.0, y = 3.0 and the momentum components described above, we obtain ˆ r p (7.4kg m 2 s )k. 3- A uniform spherical body of radius R and mass M is rotating with angular velocity Ω about an axis passing through its center. What is the weight of an object of mass m located on the surface of the sphere? Draw a diagram to clarify your answer. Ω r R MG g 2 r 2 cos rˆ R w mg θ Physics 2120 Final Examination Fall 2007 4- A thermometer of mass 55 grams and specific heat 0.837 cal/kg.K reads 15oC. It is then completely immersed in 0.3 kg of water, and it comes to the same final temperature as the water of 44.4oC. What was the initial temperature of the water? cwater=1cal/g.K. Let the initial water temperature be Twi and the initial thermometer temperature be Tti. Then, the heat absorbed by the thermometer is equal (in magnitude) to the heat lost by the water: ct mt T f Tti cw mw Twi T f . We solve for the initial temperature of the water: ct mt (T f Tti) TW i T f 44.4 o C c w mw dp where p is the pressure and V dV is the volume of the gas. For adiabatic processes in an ideal gas, show that (a) B RT B p and (b) that v s ; where vs is the speed of sound in gasses. M cp . cV (a) Differentiating Eq. 19-53, we obtain 5- The bulk modulus for gases is given as B V dp dp (constant) 1 B V (constant) dV V dV V which produces the desired result upon using Eq. 19-53 again ( p = (constant)/V). (b) Due to the fact that v B / (from Chapter 17) and p = nRT/V = (Msam/M)RT/V (from this chapter) with = Msam/V (the definition of density), the speed of sound in an ideal gas becomes v M sam / M RT / V p RT . M sam / V M 6- The temperature of one mol of a monatomic ideal gas is raised reversibly from 300 K to 400 k, keeping the volume constant. What is the entropy change of the gas? Physics 2120 Final Examination Fall 2007 Since the volume of the monatomic ideal gas is kept constant it does not do any work in the heating process. Therefore the heat Q it absorbs is equal to the change in its inertial 3 energy: dQ dEint n R dT . Thus 2 Tf 3 dQ T f 3nR 2 dT 3 J 400 K nR ln 1.00 mol 8.31 ln T i T T 2 T 2 mol K 300 K i 3.59 J/K. 7- A Carnot refrigerator extracts 35.0 kJ as heat during each cycle, operating with a coefficient of performance of 4.60. What are (a) the energy per cycle transferred as heat to the room and (b) the work done per cycle? 1 (a) Eq. 20-13 can be written as |QH| = |QL|(1 + 1/KC ) = (35)(1 + 4.6 ) = 42.6 kJ. S (b) Similarly, Eq. 20-12 leads to |W| = |QL|/K = 35/4.6 = 7.61 kJ 8- Knowing that for reference frames S and S’, moving at uniform velocity v relative to each other, x ( x vt) , show that t (t vx / c 2 ). 9- A certain particle of mass m has momentum of magnitude mc. What are (a) β, (b) γ and (c) the ration K/E0? Where K is the kinetic energy and E0 is the total energy. (a) We set Eq. 37-41 equal to mc, as required by the problem, and solve for the speed. Thus, mv mc 1 v 2 / c2 leads to 1/ 2 0.707. (b) Substituting 1/ 2 into the definition of , we obtain 1 1 v 2 / c2 1 b g 1 1/ 2 2 141 . . (c) The kinetic energy is K 1 mc 2 2 1 mc 2 0.414mc 2 0.414 E0 . which implies K / E0 0.414 . 10- For all irreversible processes involving a system and its environment: Physics 2120 Final Examination Fall 2007 a- the entropy of the system does not change b- the entropy of the system increases c- the total entropy of the system and its environment does not change d- the total entropy of the system and its environment increases e- none of the above 11- A Carnot cycle: a- is bounded by two isotherms and two adiabats on a p-V graph b- consists of two isothermal and two constant volume processes c- is any four sided process on a p-V graph d- only exists for an ideal gas e- has an efficiency equal to the enclosed area on a p-V diagram 12- A heat engine in each cycle absorbs energy from a reservoir as heat and does an equivalent amount of work, with no other changes. This engine violates: a- the zeroth law of thermodynamics b- the first law of thermodynamics c- the second law of thermodynamics d- the third law of thermodynamics e- none of the above 13- An observer notices that a moving clock runs slow by a factor of exactly 10. The speed of the clock is: a- 0.100c b- 0.0100c c- 0.990c d- 0.900c e- 0.995c Physics 2120 Final Examination Fall 2007 14- An electron is moving at 0.6c. If we calculate its kinetic energy using (1/2)mv2, we get a result which is: a- just right b- just half enough c- twice the correct value d- about 1% too low e- about 28% too low 15- A particle with zero mass and energy E carries momentum: a- Ec b- Ec2 c- Ec d- E/c e- E/c2








