template
advertisement

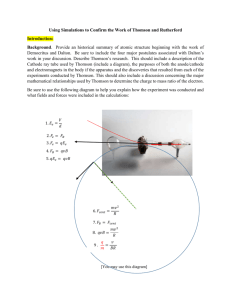
Name Date Pd Chemistry – Unit 11 – Atomic Model Research For this assignment, you will be doing some research about scientists who contributed to our current understanding of the atom. There are many scientists whose discoveries paved the way for all of us who study chemistry. You will work in groups to research. Ultimately, your goal is to complete a timeline to tell the story of the development of a model of the atom. Complete all work in your Class Notebooks. You may use the provided websites and are also encouraged to search on your own. Remember to consider the validity and reliability of internet sources. Provide citations as necessary to ensure academic honesty! For each of the following scientists you will address the following basic ideas: 1. the experimental design used (if any) 2. the evidence (data) that led to a change in the previous model 3. the problem with the previous model 4. how each scientist developed a new atomic model that a. incorporated the new evidence OR resolved a flaw in the previous model b. “invented” ideas for completeness 5. consider how other scientists contributed to the atomic model. Part 1 – J.J. Thomson (Time frame: ) 1. Describe (with diagrams & words) the experiment Thomson (and his assistants) conducted. Label a Diagram, provide a Description 2. What observable phenomena / experimental evidence did he uncover as a result of this experiment? 3. What problems did this new evidence reveal about the previous model, the Dalton Model: 4. a) Describe (in words & diagrams) the atomic model developed by Thomson to account for his experimental observations: State or come up with a nickname for this Model. b) What part of Thomson’s model directly addresses the observed phenomena? c) What part of the model was “invented” by Thomson in order to make the model complete (to make the model make sense)? 5. What did the famous “Oil Drop” Experiment of Robert Millikan add to the model? Part 2 – Ernest Rutherford (w/ Geiger & Marsden) (Time frame: ) 1. Describe (with words & diagrams) the experiment Rutherford (and his assistants) conducted. Label a Diagram, provide a Description 2. What observable phenomena / experimental evidence did he uncover as a result of this experiment? 3. What problems did this new evidence reveal about the previous model, the Dalton Model: 4. a) Describe (in words & pictures) the atomic model developed by Rutherford to account for his experimental observations: State or come up with a nickname for this Model. b) What part of Rutherford’s model directly addresses the observed phenomena? c) What part of the model (though not claimed by Rutherford!) is often attributed to this model to make it more complete.? 5. Briefly describe how the discoveries of Mosely and Chadwick added to the Rutherford Model. Modeling Chemistry 1 U10 Model Research Part 3 – Bohr (et. al.) (Time frame: ) (no part 1) 2 & 3 Describe (with words & diagrams as much as possible) what evidence and data Bohr and his colleagues soon found with the Rutherford or nuclear model. What were the major weaknesses with a nuclear model at this point? 4. a) Describe (in words & pictures) the atomic model developed by Bohr to account for his experimental observations: State or come up with a nickname for this Model. b) What part of Bohr’s model directly addresses the observed phenomena? c) What assumption did Bohr make about what electrons were doing in this model that makes the model more complete? (Bohr later rejected this assumption!) Part 4 – Timeline Put together a timeline of scientists, discoveries, and atomic models that helps you follow how our story of the atom has changed from Dalton to Bohr. Include at least the following scientists and their contributions: Scientists (not in any order….) Niels Bohr Sir James Chadwick Robert Millikan Henry Moseley Ernest Rutherford J.J. Thomson Modeling Chemistry 2 U10 Model Research