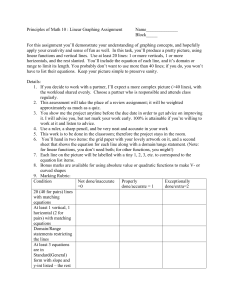
3.6 Entropy Balance Equation - TEST
advertisement

3 FUNDAMENTAL LAWS AND BALANCE EQUATIONS FOR MASS, ENERGY, ENTROPY, EXERGY, AND MOMENTUM Chapter Equation Section 3 The purpose of studying thermodynamics is to predict the behavior of systems in terms of their states as they respond to interactions with their surroundings. Classical thermodynamics is an axiomatic science; that is, the behaviors of systems can be predicted by deduction from a few basic axioms or laws, which are assumed to be always true. A law is an abstraction of myriads of observations summarized into concise statements that are self-evident and certainly without any contradiction. We have already come across the Zeroth Law of thermodynamics, which introduced temperature, a thermodynamic property, as an arbiter of thermal equilibrium between two objects. In this chapter we will introduce the conservation of mass and momentum principle, the First Law and Second Law of thermodynamics and the concept of exergy. A uniform framework in terms of balance equations will be developed. Each fundamental principle will be translated into a balance equation of a particular property. Just as equations of state are the starting point for a state evaluation, analysis of engineering systems and processes in the future chapters will begin with the balance equations. While the balance equations are derived in this chapter, their applications to closed and open systems are delegated to Chapter 4 and 5 respectively. To gain a comprehensive insight into these equations Chapters 3, 4 and 5, therefore, should be iteratively studied. 3-1 3 Fundamental Laws and the Mass, Energy, Entropy, Exergy and Momentum Balance Equations ....................................................... 3-1 3.1 Balance Equation ............................................................ 3-3 3.2 Reynolds Transport Equation (RTE) ............................. 3-5 3.3 Classification of Systems ................................................ 3-7 3.3.1 Open vs. Closed Systems ........................................ 3-7 3.3.2 Steady vs. Unsteady Systems .................................. 3-7 3.3.3 Instantaneous Rates vs. Process .............................. 3-8 3.3.4 System Tree .......................................................... 3-10 3.4 Mass Equation ............................................................... 3-10 3.4.1 Forms of Mass Balance Equation ......................... 3-11 3.5 Energy Equation............................................................ 3-12 3.5.1 Forms of Energy Balance Equation ...................... 3-14 3.6 Entropy Balance Equation ............................................ 3-16 3.6.1 Forms of Entropy Balance Equation ..................... 3-19 3.7 Exergy Balance Equation .............................................. 3-20 3.7.1 Forms of Exergy Balance Equation ...................... 3-26 3.8 Momentum Balance Equation....................................... 3-28 3.9 Balance Equations Summary ........................................ 3-30 3.9.1 General Form ........................................................ 3-30 3.9.2 Closed Systems ..................................................... 3-31 3.9.3 Closed Process ...................................................... 3-31 3.9.4 Closed Steady........................................................ 3-32 3.9.5 Open Steady .......................................................... 3-32 3.9.6 Open Process ......................................................... 3-33 3.10 Summary ....................................................................... 3-43 3.11 Index ............................................................................. 3-43 3-2 3.1 Balance Equation Each fundamental law that will be introduced in this chapter will be shown to be associated with a certain global extensive property mass m , energy E , entropy S , or momentum mVx , mV y or mVz of the system. To develop a unified framework, we will represent these extensive properties with the generic symbol B and the corresponding specific property with b . For a uniform system (Fig. 3.1), as stated in Eq. (2.94), B mb . For a non-uniform system, however, B has to be summed or integrated over the ensemble of local systems, each represented by a differential element as shown in Fig. 3.2. For a local system with a volume dV , Eq. (1.12) has to be written in a differential form. b unit of B 3 (3.1) dB bdm b dV dV unit of B = m v kg m3 /kg Integrating, b B bdm dV v unit of B (3.2) The integration is carried out over the entire system, open or closed, at a given instant. In Fig. 3.2, notice how the boundary is carefully drawn to pass through the inlet and exit ports at right angles so that two unique uniform surface states, State-i and State-e, can describe the inlet and exit conditions. Moreover, situated inside the ports slightly away from the main body of the system, these states are more likely to be uniform than if they were chosen exactly at the openings. Assuming uniformity across the inlet and exit surfaces, the flow rates of the property B at the ports can be obtained from Eq. 2.94. unit of B kg unit of B Bi mi bi ; and Be mebe = (3.3) s s kg where, mi i AV i i AV AV i i ; and me e AeVe e e vi ve kg s (3.4) Beside mass transfer, a property can be transferred across the boundary through other interactions – energy, for instance, is carried with heat and work. As will be stated shortly, entropy can not only be transferred, but also generated spontaneously within a system. Therefore, B can be expected to change with time, i.e., B B t . To use the image analogy introduced in section 1.3.3, the snapshot of the system taken with the state camera at time t provides us with the distribution of b throughout the system at that thermodynamic instant. The global property B t , therefore, can be obtained by 3-3 Fig. 3.1. A uniform system does not have to be closed. Fig. 3.2. The local system used inside the integral of Eq. (3.2). simply analyzing the picture. Similarly, a snapshot taken after an interval t can be used to evaluate B t t , the global property B at time t t . On the other hand, if the causes for a change in B t are accounted for, the change B t t - B t can be deduced entirely from a different angle. The equality between the two expressed on a rate basis constitutes the balance equation. EXAMPLE 3-1 Total Property for a Non-Uniform System. The temperature of air trapped in a vertical rigid tank of diameter 1 m and height 1 m increases linearly from 300 K at the bottom to 400 K at the top. Determine the total mass of the stratified air if the pressure inside can be assumed uniform at 100 kPa. Use the perfect gas model. SOLUTION The global properties of a non-uniform system is to be determined by treating it as an aggregate of uniform local systems. Assumptions A differential slice of air of thickness dx (Fig. 3.3) constitutes a local system in LTE. Analysis From Table C-1, obtain the necessary material properties of air: M = 29 kg/kmol, c p =1.005 kJ/kg K. The gas constant and cv are calculated (see Section 2.5.3.1.2) as R =8.314/29=0.287 kJ/kg K and cv = 1.005- 0.287 = 0.718 kJ/kg K. The variable temperature can be expressed as T c1 c2 x with c1 300 K and c2 100 K/m. Using the ideal gas equation, pv RT , Eq. (3.2) can be simplified as follows. b bp D 2 D 2 p bdx B bdm dV dx dx v RT 4 4 R 0 T 0 L L (3.5) For the evaluation of mass of the system, B m ; therefore, b 1 . Substituting this and the linear temperature relation, Eq. (3.5) can be integrated. m D2 p L L dx D2 p dx ln c1 c2 x 0 4 R 0 c1 c2 x 4 Rc2 D2 p 4 Rc2 ln T2 0.948 kg T1 TEST Solution TEST can be used only for uniform systems or binary non-uniform systems, which are made of two uniform 3-4 Fig. 3.3. A slice of air acts as a uniform local system. subsystems. Therefore, this problem, which involves an infinite number of local systems, cannot be solved using TEST. Discussion The evaluation of other properties such as energy is more complicated since b as a function of x will complicate the integrand of Eq. (3.5). For more complex systems, where variation can be in all three directions and are not known in functional terms, integration of Eq. (3.5) may be impossible. Fortunately, the global properties of non-uniform systems are seldom necessary to evaluate. Examples of property evaluation for uniform systems, which are more common, can be found in Ex. 2.19 and 2.20. 3.2 Reynolds Transport Equation (RTE) The fundamental laws are usually described with closed systems in mind. For instance, Newton’s Second Law which states that the net external force on a particle equals its rate of change of momentum, implicitly assumes the particle, the system in our case, to be closed. Similarly, the conservation of mass principle, and the First and Second Law are also easier to state as applied to closed systems. Each of these laws expresses the rate of change of a particular extensive property with respect to time in terms of other variables. In other words, a generic format for these laws can be written as dBc known (from the fundamental Law) dt unit of B s (3.6) The superscript c reminds us that this equation cannot be applied to open system as is. The right hand side (RHS) is prescribed by the specific laws to be introduced shortly. With the help of RTE the fundamental laws, which are known in the closed system format of Eq. (3.6), are expanded into balance equations applicable to any kind of system, open or closed. We begin the development of the RTE by considering a very general open system at time t and t t as sketched in Fig. 2.4. The minor restriction of a single inlet and exit will be lifted as the last step of this derivation. The system, defined by the dotted black boundary, is allowed to have all possible interactions – mass, heat and work – with its surroundings. As shown in the sketch, even the shape of the system is allowed to change. As the working substance passes through the system, we identify a closed system marked by the red boundary at time t , which occupies the entire open system plus a little region I near the inlet. The closed system becomes deformed as it flows through the open system. After a small period t , it still occupies the entire open system; however, the region-I completely disappears and a new region, region-III, not necessarily equal in size to region-I, appears near the exit. This is not a coincidence since for any given t , the region-I is carefully chosen 3-5 so that the entire fluid inside that region flows into the system during that interval. Of course, t has to be sufficiently small so as not to allow the closed system to loose its identity through disintegration, and regions I and II can be considered uniform so that BIt mIt bit , t t t t BIIIt t mIII be and (3.7) Because B is an extensive property, an inventory of B for a system can be obtained by combining contributions from different subregions comprising the system. Referring to Fig. 3.3, the change in B for the closed system as it passes through the open system (region II) can be written as B c ,t t B c ,t B t t BIIIt t B t BIt No special superscript is necessary for the open system because it is the system by default. Rearranging and substituting Eq. (3.7) t t t t B c ,t t B c ,t B t t B t mIII be mIt bit Dividing both side by t and taking a limit t t t t mIII be mIt bit Bt t Bt B c ,t t B c ,t lim lim lim lim t 0 t 0 t 0 t 0 t t t t The LHS and the first term on the RHS of this equation are clearly derivatives of the extensive property B with respect to time for the closed and open system respectively. Also, as t 0 , bet t bet , and the last two terms approach mebe and mi bi , where the superscript t is not necessary anymore since each term in this instantaneous expression refers to time t . The above equation, thus, reduces to dB c dB mebe mibi dt dt Generalizing for multiple inlets and exits, the Reynolds Transport Equation (RTE) or the general balance equation can be written as dB dt Rate of increase of B for an open system mb i i i Net flow rate of B into the system m b e e e Net flow rate of B out of the system dB c dt (3.8) Rate of increase of B for a closed system. It relates the rate of change of an extensive property B of an open system at a given instant to that of a closed system which happens to pass through with the boundaries of the two systems aligning on top of each other at that particular instant. 3-6 Fig. 3.1. A very general system at two neighboring macroscopic instants. 3.3 Classification of Systems In practical applications, thermodynamic systems or their behavior are restricted in certain ways. Therefore the general template of the balance equation, Eq. (3.8), can be simplified when applied to specific systems. For instance, if a system is closed, the mass transfer terms on the RHS drops out. In this section we will discuss, in general terms, patterns that repeat across the entire spectrum of thermodynamic devices and processes. Recognizing these patterns will help us simplify a system, classify its behavior and reduce the governing set of balance equations into custom forms. This systematic approach will be cultivated throughout this book in favor of the hit-and-miss approach of matching balance equations to specific systems that gives thermodynamics a bad name among the uninitiated. 3.3.1 Open vs. Closed Systems Classification of any system begins with the question, “Is there any mass transfer across the boundary?” If there is no mass transfer, the system is called closed. Otherwise, by default, is considered open. Obviously a system can only be open or closed, there is no other alternative. It should be stressed here that heat or work transfer has nothing to do with whether a system is open or closed. Fig. 3.5. System classification: Open vs. Closed systems. For a closed system, the mass transfer terms drop out of Eq. (3.8). 0 0 dB dBc mi bi me be ; dt dt i e dB dBc (3.9) dt dt The open system equation, thus, reduces to the fundamental laws from which they are derived. The usefulness of such an obvious equation will become clear when we introduce the individual balance equations. 3.3.2 Steady vs. Unsteady Systems A system, by default, is unsteady; that is, its global state can change with time. When the global state of a system remains frozen in time, it is said to be in steady state. In terms of our image analogy, the snapshot of a steady system does not change whether or not the system interacts with its surroundings. Hot and pressurized steam flowing into a steam turbine exits at a much lower pressure and temperature. Shaft work, flow work and even heat transfer from the turbine may occur. Yet, the turbine is most likely to operate in a steady state. At steady state all global properties, the total property B included, must remain constant since the global image does not change. Therefore, the time derivative of the LHS of Eq. (3.8) 3-7 Fig. 3.6. System classification: Steady vs. Unsteady systems. summarily drops out making the general balance equation an algebraic one. dB dt 0, Steady State mi bi mebe i e dBc dt (3.10) Obviously this simplification is applicable to both open and closed systems giving rise to four types of systems already. A closed system passing through a steady open system need not be steady. If you follow a control mass of steam as a closed system entering the turbine, it will surely undergo changes. That is why the last term in Eq. (3.10), which tracks the changes in the closed system flowing through, cannot be set to zero. In the classification process, the second question to ask is, “Does the image of the system taken with a state camera change with time?” Although the answer is a simple yes or no, sometimes it depends on the resolution or precision with which one answers the question. Inside a turbine (take a virtual tour of turbine in the TEST web site) the rotors spins at a very high RPM. Therefore, instantaneous snapshots at two different times cannot be identical. However, if the thermodynamic instant (see Section 1.3.2) is stretched by increasing the camera exposure to a few milliseconds, the pictures at two different times will be almost identical as all the fluctuations would average out in those few milliseconds. In a similar way, a car engine can be considered steady, as long as the time resolution is large enough for the piston to execute several cycles of strokes. On the other hand if we are interested in a single stroke of the piston, the picture obviously changes and the system must be considered unsteady. Fig. 3.7. As water flows through the constriction, its pressure changes. However the open system is a steady one if the global picture does not change. 3.3.3 Unsteady Process The time derivative of B is non-zero for an unsteady system. The LHS of the balance equations cannot be simplified any further if instantaneous rate of change of B is important. For example, if we are interested in the rate of change of temperature of a cup of coffee at a specific instant as it cools down, we have an instantaneous, unsteady, closed problem. The general balance equations, by default, apply to instantaneous, unsteady, open systems. Often, in unsteady systems, the change of system properties over a finite interval is of greater interest than an instantaneous rate of change. For instance, in the compression stroke of an automobile engine cycle, we are interested in the state of the gas mixture at the beginning and end of the stroke rather than at any intermediate state. Similarly, in the charging of a propane tank, another unsteady phenomenon, the instantaneous rates maybe of less significance than the overall changes during the entire process. The balance equations 3-8 Fig. 3.8. System classification: Process vs. Instantaneous rate. under such situations can be simplified by integrating with respect to time. An unsteady system is said to execute a process if it undergoes changes from a beginning global state, called the b-state or begin-state, to a final global state, called the f-state or final-state. The begin and finish states are also known as the anchor states of a process. The anchor states must be in equilibrium for a process; however, as the system moves from the b-state to f-state it does not have to pass through a succession of equilibrium for the balance equations to be simplified. For system which is uniform at the beginning and end of the process, the anchor states can be spotted on the familiar p v diagram as sketched for a compression process in Fig. 3.9. Note that without a thorough knowledge of the process, we cannot select a path between the anchor states. Fig. 3.9. In this closed process, a gas is compressed from a bState to a f-State. To identify if an unsteady system is undergoing a process, the appropriate question to ask is, “Does the unsteady system move from a clear beginning to a clear finish state?” If the answer is yes, we have a process. The simplification for a process is achieved by multiplying Eq. (3.8) with dt and integrating from the b-state to the f-state. Fig. 3.10. Inflating a tire is an open process. dBc dB mibi dt mebe dt dt dt i e finish finish finish finish finish finish finish dB c dB mi bi dt mebe dt dt dt i begin e begin begin begin dBc B f Bb mi bi dt mebe dt dt dt i begin e begin begin For an open unsteady system, the inlet and exit states are often assumed to remain uniform across the cross-section and invariant with time. The assumption, known as the uniform state and uniform flow assumption; can considerably simplify the above equation as bi and be , being independent of time, can be pulled out of the integrals. The general balance equation for an open process reduces to finish dB c B B f Bb mi bi mebe dt dt i e begin finish where, mi begin finish mi dt , and me (3.11) me dt begin 3-9 The equation still looks quite formidable with an integral of a derivative as one of its term. However, when we discuss specific balance equations, say, mass or energy equation, this term will be shown to simplify much farther. 3.3.4 System Tree The classification of systems introduced until now can be organized in a tree structure as shown in Fig. 3.11, called the system tree. The next two chapters will be devoted exclusively to the discussion of closed and open systems respectively. Further classification of closed process and open steady systems will be deferred until then. Fig. 3.11 The system classification tree. The Map in TEST displays a similar clickable tree. In TEST start at the daemons page, by using the Daemons link on the Task Bar, to classify a system. A simplification table provides links to all possible branches one can follow depending on the answer to the question posed at the table header. At any stage of simplification, a system schematic and the customized set of balance equations appear below the simplification table. Once you gain expertise in this step-by-step procedure, you can use the Map, arranged like the tree of Fig. 3.11 and linked from the Task-Bar in TEST, to jump to a specific category of systems by clicking on its node. We now begin the development of fundamental laws into balance equations and customize these equations for different classes of systems. 3.4 Mass Equation The conservation of mass principle can be stated through the following simple postulate. Fig. 3.12 Flow diagram for the mass balance equation.. Mass cannot be created or destroyed. For a closed system the total mass mc must remain constant; therefore, the time derivative of mc must be zero, i.e., dmc 0 dt (3.12) Substitute Eq. (3.12) into the RTE, Eq. (3.8), with B m and b 1 , to formulate the mass balance equation for an open unsteady system. dm dt Rate of increase of mass for an open system. m i i Net mass flow rate into the system. m e e kg s (3.13) Net mass flow rate of out of the system. 3-10 The meaning of the three terms is explained with the help of a flow diagram in Fig. 3.12. The difference between the inflow and outflow is accumulated in the balloon. Similar flow diagrams will be constructed for other balance equations. 3.4.1 Forms of Mass Balance Equation The general form of the mass balance equation can be simplified for different categories of systems classified in Fig. 3.11. Closed System Simplification For a closed system the mass transfer terms drop out. For both steady and unsteady closed systems, therefore, dm 0 or, dt m constant (3.14) This is almost a trivial result; therefore, a constant mass can be implicitly assumed for a closed system without having to refer to this equation. Open Steady Simplification As explained in section 3.3.2, at steady state the total mass, like all other global properties, remains constant. dm dt 0, steady state mi me ; i m m or, i e i e e kg s (3.15) Fig. 3.13 Flow diagram for the mass balance equation, open steady system. This form of mass conservation is often referred as “what goes in comes out”. If there is a single flow, i.e., only one inlet and one exit, the equation can be further simplified using Eq. (3.4). mi me m ; or, m i AV i i e AeVe , or m AV AV i i e e vi ve (3.16) Open Process Simplification For a process involving an open system Eq. (3.13) can be integrated or, alternatively, Eq. (3.11)can be used to produce m m f mb mi me i e finish where, mi begin (3.17) finish mi dt , and me me dt begin This form is further simplified if there is only a single inlet or a single exit as in the case of charging a propane tank or a whistling pressure cooker. Discussion of such specific cases, however, is postponed until Chapter 5. 3-11 Fig. 3.14 Flow diagram for the mass balance equation, open process. 3.5 Energy Equation The conservation of energy principle also known as the First Law of thermodynamics can be stated through the following postulates. i) The internal energy u of a system is a thermodynamic property. ii) Energy E U KE PE cannot be created or destroyed, only transferred through heat or work. On a rate basis this can be expressed as kJ s =kW dE c Qc W c dt (3.18) where, Qc is the net rate of heat transfer into the system and W c is the net rate of work or power transfer out of the system. Fig. 3.15 Flow diagram for the conservative form of the energy balance equation, open unsteady system. Substituting E , e and E c for B , b and B c respectively in the RTE and using the second postulate dE dt Rate of increase of E for an open system. m e i i i Net energy flow rate into the system. m e e e e Net energy flow rate out of the system. Q Net Rate of heat transfer into the system. W kW Net Rate of heat transfer into the system. (3.19) where, Q and W , evaluated based on the open system boundary, are substituted for Qc and W c respectively since the boundaries of the closed and open systems become coincident as t 0 . The energy flow rates at the inlet and exit can be also be expressed through the symbol E me , which is used in the flow diagram of Fig. 3.16. Equation (3.19) is now completely decoupled from the original closed system and will be labeled the conservative form of the energy equation. Different modes of heat and work transfer, shown in the flow diagram of Fig. 3.16, will be quantitatively discussed in the next chapter. As explained in Section 1.2.2.2, the transfer of heat through the ports can be neglected compared to the transfer through the rest of the boundary. The same, however, is not true about work transfer through the system ports, called the flow work. As explained in Section 1.2.2.4 different types of work transfer can be classified into two major categories, flow and external work, to distinguish open and closed systems. 3-12 Fig. 3.16 Flow diagram explaining various modes of heat and work transfer. W Net Rate of Work transfer out of the system. W F ,e i W F ,i i Net Flow Work Out Net Flow Work In Wsh Wel WB Shaft Work Out Electrical Work Out Boundary Work Out Other Work, WO Flow Work, WF (3.20) WF WO WB WF Wext External Work, Wext For a closed system WF 0 and there is no distinction between W and Wext . To evaluate the flow work, consider the small fluid element of length xe in the simplified system of Fig.3.17 that is pushed out of the system by the pressure force from the left against the pressure from the right. The pressure force Fe pe Ae does a work of Fe xe (see Section 1.2.2.3) in t . According to the sign convention, the exit work must be positive since work is done by the system. In a similar manner, as a fluid element is pushed into the system against the resistance of the inlet pressure, a negative work transfer with a magnitude of Fi xi takes place in time t at the inlet. As t 0 , the net flow work rate or flow power can be written with the help of Eq. (3.16) as Fe xe Fi xi pe Ae xe pi Ai xi t t t t (3.21) AeVe AV i i pe AeVe pi AV pe ve pi vi me pe ve mi pi vi i i ve vi Fig. 3.17 A fluid element at the exit being expelled by the system against an external pressure. WF WF ,e WF ,i A port with a very small area still can have very large pv and, thus, transfer a relatively significant amount of flow work. Equation (3.21) can be generalized for multiple inlets and exits. WF me pe ve mi pi vi e flow energy J is equivalent to the flow of energy E and the transfer of flow work WF across a control (3.22) i Each term on the RHS resembles flow rate of properties discussed in Section 2.8. The flow work too, therefore, can be regarded as a flow property. Substituting the above expression for flow work after separating it from all other work terms, the conservative form of the energy equation, Eq. (3.19), can be rewritten as dE mi ei pi vi me ee peve Q Wext dt i e Fig. 3.18 The flow of (3.23) In this modified form the mass flow can be seen to carry a combination property consisting of energy e and a term that 3-13 surface. represents the flow work performed per unit mass of the flow. We call this combination property the specific flow energy and represent it with the symbol j in the absence of any universally accepted symbol for this important convenience property. j e pv u ke pe pv h ke pe (3.24) Substituting the symbol j for the specific flow energy, we obtain the balance equation for energy in its most general form. dE dt Rate of increase of E for an open system. m j i i m j e e i e Net flow rate of flow energy into the system. Net flow rate of flow energy out of the system. where, j h ke pe h Q Net Rate of heat transfer into the system. kW Wext Net Rate of work transfer into the system. V2 gz , and Wext WB WO 2000 1000 (3.25) The energy carried by the flow E me in the conservative form, Eq. (3.19), is replaced in this equation by the flow energy carried by the flow, J mj . The advantage of this form is that only the readily recognizable external work appears in this equation and the hidden work of flow can be completely ignored since it is already accounted for in the use of the property j . It may seem that this form of energy equation is meant only for open systems. To the contrary, if we Fig. 3.19 By using specific flow energy j instead of specific energy e , the cumbersome flow work can be forgotten. 0 substitute WF 0 and W WF Wext Wext into Eq. (3.25), the second postulate of the First Law is immediately recovered making Eq. (3.25) the most general form from which all other forms should be derived. The meaning of various terms in this equation is explained through the flow diagram of Fig. 3.18. 3.5.1 Forms of Energy Balance Equation As we did with the mass balance equation, the energy equation is customized for the particular classes of systems introduced in the system tree of Fig. 3.11. Closed System Simplification For a closed system the mass transfer terms drop out and Wext W as there is no possibility of any flow work. The energy balance equation, Eq. (3.25), reduces to the second postulate of the First Law. dE Q W dt (3.26) Obviously, this forms suits any instantaneous unsteady closed system. There is no need for the superscript c anymore because we 3-14 Fig. 3.20 For a closed system there is no flow work; therefore, W Wext . are deriving a restricted form from a more general form applicable to both open and closed systems. Closed Process Simplification For an unsteady closed system going through a process, Eq. (3.26) can be integrated from the b-state to the f-state as outlined in section 3.3.3 producing Fig. 3.21 Energy flow diagram for a closed process. E E f Eb Q W finish where, Q finish Qdt , and W begin finish WB dt begin WO dt WB WO (3.27) begin This is an algebraic equation that relates two anchor states through two process variables Q and W . Closed Steady Simplification For a steady system, open or closed, the time derivative of any global property must be zero. The energy equation, thus, simplifies to Q W (3.28) Fig. 3.22 Energy flow diagram for a closed steady system. The net rate of heat transfer to a steady closed system must be exactly equal to the net rate of work delivered by the system. Open Steady Simplification The time derivative of all global properties of the system must be zero at steady state as the global picture remains frozen at steady state. The energy equation simplifies to what is commonly called the steady flow energy equation (SFEE). 0 mi ji me je Q Wext i (3.29) Fig. 3.23 Energy flow diagram for an open steady system. e By rearranging the equation, it can be shown that the sum total of the rate of flow of flow energy and heat into a steady open system must be equal to the rate at which energy leaves the system through flow energy and external work. Like the steady state mass balance equation, it expresses what goes in, comes out in terms of energy. Open Process Simplification For a process involving an open system Eq. (3.26) can be integrated from the begin to the finish state as outlined in section 3.3.3 for a generic property. Using the uniform flow uniform state assumption, the energy equation reduces to E E f Eb mi ji me je Q Wext i e finish where, Q begin (3.30) finish Qdt , and Wext Wext dt begin 3-15 Fig. 3.24 Energy flow diagram for an open process. The mass transfers in such a process has already been examined in section 3.4.1. 3.6 Entropy Balance Equation The Second Law of thermodynamics can be stated through the following postulates. i) Entropy S is an extensive property that measures the degree of disorder in a system. The specific entropy s is a thermodynamic property. ii) Entropy can be transferred across a boundary through heat but not through work. The rate of entropy transfer by Q crossing a boundary at a temperature TB is given as Q / TB . iii) Entropy cannot be destroyed. It can be generated by natural processes, i.e., Sgen 0 . iv) An isolated system achieves thermodynamic equilibrium when the entropy of the system reaches a maxima. Let us go over these statements one at a time. From our experience of chaos, we would tend to agree with the first postulate that entropy, being a measure of total amount of chaos or disorder in a system, is an extensive property; that is, doubling the size of a uniform system will double its entropy. Heat transfer to a system can be expected to increase the molecular disorder and, hence, entropy. If a uniform system is at a high temperature and, therefore, pretty chaotic to start with, addition of heat cannot be expected to add as much entropy to the system as would be the case for a cooler, less chaotic system. This provides justification as to why the boundary temperature, which is same as the system temperature for a local system, occurs in the denominator of the entropy transfer term in postulate-II. Observe that transfer of work does not seem to affect entropy of a system. Work involves organized motion such as the rotation of a shaft, motion of a boundary, and, in the case of electricity, directed movement of electrons, etc. The chaotic motion of the system, therefore, remains unaffected by the transfer of organized motion. The third postulate states that every system has a natural tendency towards generating entropy. Because entropy cannot be destroyed, the generated entropy is a permanent signature of the process. When heat radiates from the Sun to earth, the coffee in the stirred cup gradually comes to rest, electrons flow across a voltage difference, a drop of ink dissipates in a bucket of water, rubbing one hand against another make them warm, natural gas burns in air forming hot flames, a volcano erupts – there is one thing that is 3-16 Fig. 3.25 CARTOON Are you saying that the Second Law left those footprints? common in all these apparently unrelated phenomena; they all tend to destroy a gradient of some kind while generating entropy as dictated by postulate-II. In the next chapter we will devote an entire section going after these sources of spontaneous entropy generation. For the time being, we will refer to all these gradient destroying natural phenomena as generalized friction. Generalized friction leave an indelible footprint in the form of entropy generation. Any process involving generalized friction, therefore, cannot be completely reversed and are called irreversible, the degree of irreversibility being proportional to the entropy generation. Generalized friction due to system surroundings interactions sometimes extends beyond the system into the immediate surroundings. Depending on the location where the entropy is generated with respect to the system boundary, the associated irreversibilities are called internal if within the system and external if outside or at the boundary. For instance, entropy is generated inside and in the immediate surroundings of a turbine operating in a steady state. The system’s universe enclosed by the outer boundary of Fig. 3.26 includes both the internal and external generation of entropy. In the limiting situation of no entropy generated in the system’s universe as a result of a particular process, the system can be completely restored back to its original state without leaving any clue that the original process ever took place. The system or process is said to be reversible under that ideal situation. The concept of entropy generation will be linked in the next chapter with the design of more efficient engines, refrigerators and various other thermal devices. The third postulate (not to be confused with the Third Law of thermodynamics to be introduced in Chapter-8) has tremendous implications in predicting equilibrium, which will be discussed in more details in Chapter 8 and 10. For the time being, consider two closed insulated systems, initially at two different temperatures, brought in diathermal contact by removing insulations from two walls and pressing the two blocks against each other on their uninsulated faces. The entropy of the combined system will start to increase as entropy is generated due to heat transfer from the hotter block to the colder one. We know from our experience that at equilibrium temperatures of the two blocks will become equal, at which point entropy will cease to increase any further, all the temperature gradient having been completely destroyed. Thus entropy has been maximized as the isolated system, consisting of the two blocks, comes to equilibrium. As a matter of fact, we will show in Chapter-8, that starting from the second law, the equality of temperature at equilibrium can be predicted. Although this may seem like a trivial exercise, the same principle will help us deduce in 3-17 Fig. 3.26 Entropy is generated in the shaded area which extends beyond the system boundary. Fig. 3.26 The interactions between the system and its surroundings causes entropy generation inside and in the immediate surroundings of a system. Chapter –10, the emissions from combustion, something far from trivial. Getting back to our task of translating the fundamental laws into balance equations, the second postulate can be written as. dS c Q c c Sgen ; dt TB kW K c Sgen 0 (3.31) where, Sgen is the rate of entropy generation within the closed system boundary and Qc is the rate of heat transfer into the closed system of Fig. 3.4. Substituting S and s for B and b respectively in the RTE, we obtain the general entropy balance equation. dS dt Rate of increase of S for an open system. m s i i i Net flow rate of entropy into the system. where, m s e e e Net flow rate of entropy out of the system. Q TB Net Rate of entropy transfer through heat. Sgen Net Rate of generation of entropy inside the system. kW K (3.32) Sgen 0 As mentioned before, the boundary of the closed system passing through the open system of Fig. 3.4 is almost identical to that of the c open system as t goes to zero. Therefore, Q Qc and Sgen Sgen . The comments under each term are keyed to the open system of Fig. 3.4 as this general entropy equation completely stands on its own without any reference to the closed system to which it owes its origin. The flow diagram of Fig. 3.27 also explains the various terms of the entropy equation. An arrow with dots inside is used to signify the generation of entropy. For most systems on earth, the heat interaction takes place with the surrounding atmosphere. If the system boundary is carefully drawn to pass through the surrounding air, atmospheric temperature can be used for TB . Obviously the precise location of the boundary does not affect Q or W , which are flow rates of energy; however, being a cumulative quantity, Sgen depends entirely on the selection of boundary. The total rate of entropy generation in the turbine of Fig. 3.26, for instance, can be expressed as the sum of the entropy generation inside the system and in the immediate surroundings external to the system. Sgen,univ Sgen,int Sgen,ext kW K (3.33) where the subscript univ is used to signify the system’s universe. 3-18 Fig. 3.27 Entropy is accumulated due to generation and transfer through mass and energy. Fig. 3.28 Sgen,univ Sgen Sgen,ext includes all sources of entropy generation inside and outside the system. If a system exchanges heat with different segments of the surroundings at different temperatures as shown in Fig. 3.28, the boundary of the extended system can be made to pass through k segments each at a uniform temperature Tk . The entropy balance equation for the system’s universe modifies as follows Q dS kW mi si me se k Sgen,univ dt K i e k Tk Fig. 3.29 Flow diagram of entropy for an extended system with surroundings at two different temperatures. (3.34) The total entropy S , the mass flow rates mi or me and the heat transfer rate Qk are assumed not affected significantly by extending the system to include the thin layer of immediate surroundings. The entropy generation, however, can be huge outside the system, even in a very thin layer. This will be discussed with examples in the next chapter. 3.6.1 Forms of Entropy Balance Equation As we did with the mass and energy balance equations, we will customize the entropy equation in a similar manner for different classes of systems. Although the following equations are written for a system with a fixed boundary temperature TB , they can be modified for an extended system by replacing Sgen with Sgen,univ and Qk T k Fig. 3.30 Entropy flow diagram for a closed, instantaneously unsteady system. Q with TB . k Closed System Simplification For a closed system the mass transfer terms drop out and Eq. (3.34) reduces to dS Q Sgen dt TB (3.35) Obviously, this form suits any instantaneous unsteady closed system. Closed Process Simplification For an unsteady closed system going through a process, Eq. (3.35) can be integrated from the b-state to the f-state as outlined in section 3.3.3 producing S S f Sb Q Sgen TB finish where, Q begin (3.36) finish Qdt , and Sgen Sgen dt begin This is an algebraic equation that relates two anchor states through two process variables Q and S gen . Obviously Sgen 0 since Sgen 0 3-19 Fig. 3.31 Entropy flow diagram for a closed, process. Closed Steady Simplification For a steady system, the time derivative of any global property must be zero. Eq. (3.35) simplifies to Q S gen TB 0 (3.37) Fig. 3.32 The default direction of heat flow is inconsistent with the direction of entropy transfer. A number of Second Law statements can be deduced from this equation in the next two chapter. Open Steady Simplification With the time derivative of S disappearing at steady state, the entropy equation, Eq. (3.32), simplifies to a form similar to the steady flow energy equation. 0 mi si me se i e Q Sgen TB (3.38) Note that unlike the mass or energy equation, the entropy equation cannot be rearranged in the what-goes-in-must-come-out format. Because of entropy generation, what comes out is often more that what goes in. Open Process Simplification For a process involving an open system, Eq. (3.32) can be integrated from the begin to the finish state as outlined in section 3.3.3. Using the uniform flow uniform state assumption, the entropy equation reduces to S S f Sb mi si me se i i finish where, Q Q Sgen TB finish Qdt , and Sgen begin Fig. 3.33 What comes out may be more than what goes in since Sgen 0 . Fig. 3.34 Entropy diagram for an open process. (3.39) Sgen dt begin The mass transfers in such a process has already been examined in section 3.4.1. 3.7 Exergy Balance Equation Stagnant air and wind both have energy. Yet it is much easier to extract useful work out of the wind than stagnant air at atmospheric conditions. One of the major quests for engineers at all times has been delivery of useful work in the form of shaft or electrical power out of any source of available energy - wind, ocean waves, river streams, geo-thermal reserves, solar radiation, fossil fuels, nuclear materials, etc. With the help of the mass, energy and entropy balance equation we are about to predict the maximum possible useful work that can be extracted from a system. In fact manipulation of these equations will be shown to lead to a new 3-20 Fig. 3.35 CARTOON – A ship stuck in an ocean or a car stuck in a desert. Energy Energy, every where but not a drop of exergy to drink. balance equation for a new property called exergy, which measures the useful energy content of a system. The ocean or the atmosphere may have tremendous amount of energy due to their huge mass alone, but very little exergy; that is why, a ship or an airplane cannot extract any useful work out of these reservoirs of energy. Exergy, thus, can be looked upon as a measure of the quality of energy. An exergy analysis not only helps in comparing two alternative sources of energy - or should we say exergy – but also in rating the performance of any device that consumes or delivers useful work. Because exergy is a derived concept, we must develop the balance equation first before identifying the different mechanisms of its storage, transfer and generation, if any. Only then can we precisely define exergy as a property. Consider the system schematic sketched in Fig. 3.35. Like Fig. 3.4, it has only one inlet and one exit, a restriction that will be readily removed towards the end of our derivation. The surroundings, however, is divided into two thermal energy reservoirs or TERS – a thermal energy reservoir is a large system whose temperature is assumed not affected by heat transfer –reservoir 0, which is atmospheric air at a standard temperature T0 and reservoir k , say, a furnace at a temperature Tk . Note that a TER is sometime called a heat reservoir, an oxymoron for a form of energy which can only be transferred and not stored. Obviously, the number of TERS can be extended just the same way as the number of inlets or exits. For the extended system enclosed by the boundary of Fig. 3.35, the energy and entropy equations can be written as dE mi ji me je Qo Qk Wext dt (3.40) Q Q dS mi si me se o k Sgen,univ dt To Tk (3.41) By including the immediate surroundings, we capture the entire amount of entropy generation Sgen,univ (see Eq. (3.33))due to the interactions between the system and its surroundings; moreover, the choice of the boundary temperature is simplified as it passes through the TER’s with constant temperatures T0 and Tk . Note that variables, mi , me , ji , je , si , se , Qo , Qk , or Wext are unaffected by the choice of the extended system. If the mass (or thermal capacity) of the added layer of immediate surroundings can be considered small compared to the internal system, E and S also can be assumed identical between a system and its extended version. The 3-21 only difference, therefore, comes from the entropy generation contributed by the immediate surroundings. Multiplying Eq. (3.41) by T0 , a constant, and subtracting the resulting equation from Eq. (3.40) we get d E T0 S mi ji T0 si me je T0 se dt T Qk 1 0 Wext T0 Sgen,univ Tk (3.42) Before we proceed further, let us take a closer look at the external work transfer. Different modes of work transfer have been introduced in a qualitative manner in Section 1.2.2.4 and through a flow chart in Fig. 3.16, which is further modified in Fig. 3.37. Most components of the external work transfer, electrical, shaft or power transfer through a piston into the crank shaft are readily useful. The only exception is the part of boundary work transfer that involves the atmosphere. The work done by the piston in pushing the atmospheric air in Fig. 3.36 cannot be used for any practical purpose. Similarly if the piston is pushed downward, the atmospheric work comes free and does not cost anything as does any other form of useful work. The external work term in Eq. (3.42), therefore, can be separated into an useful and atmospheric components as shown by the flow diagram of Fig. 3.37. Wext Wu Watm Fig. 3.37 Different modes of external work in the energy flow for the system in Fig. 3.36. (3.43) Although evaluation of boundary work is a topic for the next chapter, we can evaluate the atmospheric work transfer due to the displacement x of the piston in time t as shown in Fig. 3.38 by recalling the definition of work (see Section 1.2.2.3) and the sign convention. Watm lim t 0 p0 Ax lim t t 0 p0 V d p0 V dV p0 t dt dt (3.44) The inner negative sign accounts for the fact that the atmospheric force and the displacement of its point of application are in opposite direction. The outer negative sign converts the work done by the atmosphere into work done by the system. The expression derived for a single piston can be shown to remain valid even if the entire boundary of a system is non-rigid. The boundary, in that case, can be divided into a large number of discrete pistons-cylinder arrangements and the contributions from individual elements when integrated can be shown to produce the same result as Eq. (3.44). Of course, the expression derived for the atmospheric work is valid for both expansion or contraction of the system. 3-22 Fig. 3.38 Evaluation of atmospheric work transfer. Using Eqs. (3.44) and (3.43), Eq. (3.42) can be rearranged as d E T0 S p0 V mi ji T0 si me je T0 se dt B A T Qk 1 0 Tk T0 Sgen,univ Wu D kW (3.45) E C Before we manipulate this equation any further, let us introduce the concept of the dead state. Work is generally extracted during gradient destroying natural process – a hydraulic power plant needs a difference in water height, a wind turbine requires a velocity difference between the wind and the rotor blades, a thermal power plant requires a temperature difference between the boiler and the atmosphere, to name a few. When a system comes to thermodynamic equilibrium with quiescent atmospheric air at sea level, there is no mechanical, thermal or chemical driving force left for it to interact with the surroundings. In other words, there is no juice or exergy left in the system to extract useful work out of. Such a state with V 0 , z 0 , T T0 and p p0 is said to be at its dead state. Getting back to the Eq. (3.45), if the stream flowing through the system is brought to equilibrium with the surrounding air at p0 and T0 , the combination property j T0 s carried by the stream reduces to j T0 s 0 0 0 j0 T0 s0 h0 ke0 pe0 T0 s0 h0 T0 s0 (3.46) It should be emphasized that at the dead state the working substance that make up the system is at p0 and T0 with no kinetic or potential energies. However, other specific properties such as h0 , s0 , etc., are properties of the working substance of the system, and are not necessarily equal to that of the surrounding air. With the help of Eq. (3.46), the term B of Eq. (3.45) can be modified by using the dead state as a reference state for the combination property j T0 s . A mi ji T0 si j0 T0 s0 me je T0 se j0 T0 s0 F j0 T0 s0 mi me CDE kW G (3.47) H The combination property carried by the mass flows mi and me in this equation is called the specific flow exergy and is represented by 3-23 Fig. 3.39 CARTOON You got a dead battery, Madam! A system in equilibrium with the quiescent ambient atmosphere is said to be at its dead state. the symbol . Defined in terms of intensive property T0 , and specific properties j and s , j T0 si j T0 s0 must be an extrinsic specific property since j is an extrinsic property. It has a zero value at the dead state and has the same unit as j , i.e., kJ/kg. The physical meaning of flow exergy will be discussed shortly and plenty of examples will be covered in Chapter 6. Term H in the above equation can be further simplified by employing Eq. (3.46) and the mass balance equation, Eq. (3.13). dm dt dH 0 dS d d mh0 T0 ms0 T0 0 dt dt dt dt d d H 0 T0 S0 U 0 p0 V0 T0 S0 dt dt d E0 T0 S0 p0 V dt H j0 T0 s0 mi me h0 T0 s0 Taking the term H into the LHS of Eq. (3.47), A-H d E T0 S p0 V E0 T0 S0 p0 V0 dt (3.48) The combination extensive system property that appears inside the time derivative is now referenced at the dead state, i.e., its value at the dead state is zero. This is called the exergy of the system and has the same unit as E , i.e., kJ. The total exergy is represented by the symbol (pronounced phi) and the corresponding specific property by , and are defined in Eq. (3.49). While is a total extensive property, is an extrinsic property because it has extrinsic components such as ke and pe. Substituting Eq. (3.48) into Eq. (3.47), and generalizing the number of inlets, exits and TER’s, we obtain the general exergy balance equation . 3-24 d dt m i i i Rate of increase of total exergy of an open system. Net flow rate of flow exergy into the system. Wu m e e Net flow rate of flow exergy out of the system. Net Rate of exergy transfer through heat. kW I Rate of irreversiblities or exergy destruction due to entropy generation. Rate of exergy transfer through useful external work. where, T Qk 1 0 k Tk e dV E T S p V E 0 0 0 T0 S0 p0 V0 sys (3.49) U U 0 T0 S S0 p0 V V0 KE PE u u0 T0 s s0 p0 v v0 ke pe j T0 s j0 T0 s0 V2 gz h h0 T0 s s0 2000 1000 I T0 Sgen,univ Although the equation looks formidable at first sight, it lends itself to interpretation just like all other balance equations derived so far. The LHS, as is usual, represents the time rate of increase of an extensive property, the system exergy in this case. Like any other total property of a non-uniform system (see Eq. (3.2)) it can be obtained by integrating or summing up the specific exergy over the local systems comprising the global system. The RHS lists all possible ways that affect the stored exergy of a system. Flow exergy, just like flow energy j , is carried by the flow in and out of the system. Heat transferred into the system from a TER at Tk T0 carries a fraction, 1 T0 / Tk , of itself as exergy. Note that for reservoir k 0 , i.e., the ambient atmosphere, this fraction reduces to zero. That is, there is no exergy is transferred through heat transfer between the system and the ambient atmosphere. The implication of a cold reservoir, i.e., Tk T0 , will be discussed in Section 4.3.3.4. The exergy delivered by the system as useful work, Wu , appears with a negative sign as it drains the system of its stored exergy. Finally, the entropy generation can be seen to produce a term that must be always non-positive since Sgen,univ 0 (Second Law). It is called the rate of exergy destruction or the rate of irreversibility and is represented by the symbol I . 3-25 Fig. 3.40 Flow diagram of exergy for an extended system. Each term of this equation sketched in the flow diagram of Fig. 3.40 will be explained with plenty of examples in the next two chapters. A comparison of the flow diagrams for energy (Fig. 3.15), entropy (Fig. 3.27) and exergy (3.40) can be helpful in understanding the similarities and differences in the inventory of the three properties in terms of a common framework. 3.7.1 Forms of Exergy Balance Equation As we did with the mass, energy and entropy balance equations, we will customize the exergy equation in a similar manner for different classes of systems. Closed System Simplification For a closed system the mass transfer terms drop out and Eq. (3.49) reduces to T d Qk 1 0 Wu I dt k Tk Fig. 3.41 A smart coffee mug that produces electricity as the coffee cools down to room temperature. (3.50) Obviously, this form suits any instantaneous unsteady closed system. Closed Process Simplification For an unsteady closed system going through a process, Eq. (3.50) can be integrated from the b-state to the f-state as outlined in Section 3.3.3 producing T f b Qk 1 0 Wu I k Tk finish where, Qk finish Qk dt , Wu begin (3.51) Wu dt and, I T0 S gen,univ begin The simplified form of the exergy equation for a closed process can be used to explore the physical meaning of some of its terms. For instance, when a closed system, say, a warm cup of coffee cools down from a temperature Tb to the room temperature T0 by rejecting Qloss amount of heat, no useful work is produced. However, the exergy equation can be used to see if it is possible to construct a clever device to extract useful work out of this cooling process. With Tk T0 , Eq. (3.51) simplifies as Wu b f I (3.52) Clearly it is possible to convert some of the exergy in a coffee mug into useful work. If the final state is the dead state, i.e., the coffee in the mug reaches equilibrium with the environment, f 0 . Being a non-negative quantity, the irreversibility I can be seen to reduce the useful work output. In fact for a regular coffee cup, the exergy is 3-26 Fig. 3.42 The exergy of a warm coffee mug is the maximum possible useful work that can be extracted as the coffee comes to equilibrium with the surrounding air. Fig. 3.43 Energy flow diagram for Eq. (3.54). The direction of the heat arrow is reversed since Q Qloss ( Qloss is a positive quantity). completely destroyed by I . If the irreversibility can be eliminated and the Second Law does permit Sgen,univ 0 as a limiting ideal case - the work produced is maximized. Wu ,max b f 0 I 0 (3.53) The exergy of a system, therefore, has the simple interpretation of the maximum possible useful work that can be extracted out of it by transferring heat with only the atmospheric TER. One may naturally ask, why cannot we use an energy analysis instead to predict the maximum work transfer? The next chapter will be devoted to analysis such as this for closed system. As a preview let us see what the energy and entropy equation predict about the system at hand. Using the solid/liquid model for the coffee, the energy equation, Eq. (3.27), can be simplified as E U f U b KE PE Q W Qloss W 0 0 W U b U f Qloss mcv Tb T0 Qloss (3.54) By eliminating Qloss completely it seems that the change in internal energy can be completely converted into work, i.e., Wmax mcv Tb T0 . The Second Law however has been completely disregarded in arriving at this conclusion. In fact, an entropy equation for the process, Eq. (3.36), yields S S f Sb Sgen,univ Qloss S Q Sgen gen,univ TB T0 T Q mcv ln 0 loss Tb T0 (3.55) The first term on the RHS being negative, an elimination of Qloss would result in a negative S gen,univ , which is a direct violation of the Second Law. Any conclusions from the energy equation, therefore, must be tested for compliance with the Second Law. Conclusions derived from the exergy balance equation, on the other hand, do not run into these types of difficulty as the exergy equation is firmly rooted in the combination of mass, energy and entropy equations. Closed Steady Simplification For a steady system, the time derivative of , a global property, is set to zero and Eq. (3.50) simplifies to T 0 Qk 1 0 Wu I k Tk (3.56) 3-27 Fig. 3.44 The change in U and S according to the solid/liquid model as the temperature goes from Tb to T0 . Mass of the cup is neglected in these expressions. Open Steady Simplification The steady state exergy equation, similarly, can be expressed in an algebraic form as the time derivative drops out. T 0 mi i me e Qk 1 0 Wu I i e k Tk (3.57) The destruction of exergy term makes it impossible to express this equation in the what-comes-in-must-go-out format. To explore the physical meaning of flow exergy, consider a steady stream of fluid flowing through a system which has heat interactions with only the atmospheric reservoir. The power delivered by this device can be obtained from Eq. (3.57) as Wu mi i me e I mi i e I (3.58) The useful work is maximized when the exergy destruction is eliminated and the flow exits at its dead state. 0 0 Wu ,max mi i me e I mi i i (3.59) The flow exergy, therefore, can be interpreted as the maximum possible useful work delivered per unit mass of the flow if the flow is brought to dead state by exchanging heat with the atmospheric TER. Complete analysis of open systems will be carried out in Chapter 5 at which point this will be a simple exercise to show that a First Law analysis alone cannot be used for predicting the maximum work transfer since the Second Law may be violated. Open Process Simplification For a process involving an open system Eq. (3.49) can be integrated from the begin to the finish state. Using the uniform flow uniform state assumption, the exergy equation reduces to T f b mi i me e Qk 1 0 I i i k Tk (3.60) where many of the symbols have been explained in connection with the corresponding form of the energy and entropy equations. 3.8 Momentum Balance Equation The momentum equation will not be used until chapter 7, where we will discuss modern jet engines. However, this is the appropriate place to cast Newton’s law into our common framework of a balance equation that applies to all systems, open or closed. 3-28 Fig. 3.45 Flow diagram of exergy simplified for an open steady system. Newton’s Second Law of Motion for a closed system can be stated as The rate of change of momentum of a closed system is equal to the net external force applied on the system. Because momentum and force are vectors, the momentum equation can be split into three independent equations along x , y , and z directions in the Cartesian coordinates. Along the x direction, Newton’s Second Law can be written as dM xc Fxc dt kN; where, M x mVx 1000 kN kg.m kN.s= N s3 s (3.61) Observe that in this equation the unit of force is kN to be consistent with all other balance equations and the unit of pressure, a deviation from the standard use of N in mechanics. Substituting M x and Vx /1000 for B and b respectively in the RTE, Eq. (3.8), we obtain the general momentum balance equation. dM x dt Rate of increase of x -momentum of an open system. mV i x ,i i /1000 meVx ,e /1000 + Net x -momentum flow rate into the system. i Net x -momentum flow rate out of the system. F kN (3.62) x Net Rate of generation of x -momentum. As in the energy and entropy equation, the superposition of the closed and open system is exploited to substitute Fx Fxc . Like any other extensive property, momentum can be transported in and out of the system with mass. Like the entropy generation term in the entropy equation, the net external force acts as a source of momentum. For closed systems, Newton’s law of motion is recovered. d mVx /1000 dM x max Fx or, Fx or, Fx (3.63) dt dt 1000 where, ax is the acceleration in the x direction. For an open steady system Eq. (3.62) reduces to 0 1 mV i x ,i meVx ,e Fx 1000 i i (3.64) These are the only forms of the momentum equation that will be used in Chapter 7 and 11, although other forms can be derived as easily. 3-29 Fig. 3.46 An external force is necessary to balance the momentum flow. The momentum equation in the y or z directions can be written by simply changing the subscript x into y and z respectively. 3.9 Balance Equations Summary The complete set of governing balance equations are summarized below for selected categories of systems that will be frequently encountered in the rest of the chapters. Although momentum equation is also included, often the MEEE equations -the mass, energy, entropy and exergy equations -constitute the core governing balance equations in thermodynamic problems. 3.9.1 General Form The following are the balance equations for open and unsteady systems. All other forms can be derived from this equation set. Mass (Eq. (3.13)) dm mi me dt i e kg s (3.65) Energy (Eq. (3.25)) dE mi ji me je Q Wext dt i e where, j h ke pe h kW (3.66) 2 V gz ; Wext WB WO 2000 1000 Entropy (Eq. (3.32)) dS Q kW mi si me se Sgen dt TB K i e (3.67) Exergy (Eq. (3.49)) T d mi i me e Qk 1 0 dt i e k Tk Wu I where, E T0 S p0 V E0 T0 S0 p0 V0 kW (3.68) j T0 s j0 T0 s0 I T0 Sgen,univ Momentum (Eq. (3.62)) 3-30 Fig. 3.46.1 System schematic to accompany Section 3.9.1. dM x 1 mV i x ,i meVx ,e Fx dt 1000 i i mVx where, M x 1000 kN kN kg.m kN.s= N s 2 s (3.69) 3.9.2 Closed Systems Considerable simplification results as the mass transfer terms are dropped from the balance equations for closed systems. Moreover, flow work being completely absent, W Wext . Mass (Eq. (3.13)) dm 0 dt kg s ; m constant kg (3.70) Energy (Eq. (3.25)) dE Q W Q WB WO dt kW ; (3.71) Entropy (Eq. (3.32)) dS Q kW Sgen dt TB K (3.72) Fig. 3.46.2 System schematic to accompany Section 3.9.2. Exergy (Eq. (3.49)) T d Qk 1 0 Wu I dt k Tk kW (3.73) M x constant (3.74) Momentum (Eq. (3.62)) dM x Fx dt kN ; 3.9.3 Closed Process When an unsteady closed system undergoes a change of state from a begin-state to a finish-state, it is said to have executed a closed process. Mass (Eq. (3.14)) 3-31 m constant (3.75) [kg] Energy (Eq. (3.27)) E E f Eb Q W Q WB WO Entropy (Eq. (3.36)) S S f Sb Q Sgen TB (3.76) Fig. 3.46.3 System schematic to accompany Section 3.9.3. (3.77) Exergy (Eq. (3.51)) T f b Qk 1 0 Wu I k Tk (3.78) 3.9.4 Closed Steady When the image of a closed system taken with a state camera does not change with time, the time derivative of all global properties becomes zero and the system is said to be a closed steady system. Closed cycles, as will be shown in the next chapter, can be treated as a special case of a closed steady system. Mass (Eq. (3.14)) m constant (3.79) Energy (Eq. (3.28)) 0 Q W (3.80) Entropy (Eq. (3.37)) 0 Q Sgen TB T 0 Qk 1 0 Wu I k Tk Exergy (Eq. (3.56)) Fig. 3.46.4 System schematic to accompany Section 3.9.4. (3.81) (3.82) 3.9.5 Open Steady When the image of an open system taken with a state camera does not change with time, the time derivative of all global properties becomes zero and the system is said to be an open steady system. Mass (Eq. (3.15)) 0 mi me i e kg s (3.83) Energy (Eq. (3.29)) 3-32 Fig. 3.46.5 System schematic to accompany Section 3.9.5. 0 mi ji me je Q Wext i [kW] (3.84) kW K (3.85) kW (3.86) e Entropy (Eq. (3.38)) 0 mi si me se i e Q Sgen TB Exergy (Eq. (3.57)) T 0 mi i me e Qk 1 0 Wu I i e k Tk Momentum (Eq. (3.64)) 0 1 mV i x ,i meVx ,e Fx 1000 i i kN (3.87) 3.9.6 Open Process When an unsteady open system undergoes a change of state from a begin-state to a finish-state, it is said to have executed an open process. The inlet and exit states are carefully chosen so that their properties can be assumed to remain unchanged over time and over the cross-sectional areas. This is known as the uniform state uniform flow assumption. Mass (Eq. (3.17)) m m f mb mi me ; i (3.88) e Energy (Eq. (3.30)) E E f Eb mi ji me je Q Wext i (3.89) e Entropy (Eq. (3.39)) S S f Sb mi si me se i i Q Sgen TB (3.90) Exergy (Eq. (3.60)) T f b mi i me e Qk 1 0 I i i k Tk (3.91) 3-33 Fig. 3.46.6 System schematic to accompany Section 3.9.6. EXAMPLE 3-2 MEEE Equations for a Closed Process. Develop the appropriate form of MEEE (mass, energy, entropy and exergy) equations for the following problem. Determine the amount of heat necessary to raise the temperature of 1 kg of aluminum from 30 o C to 100 o C ? SOLUTION The customized form of balance equations for various classes of systems have been already identified in this chapter. Therefore, the task at hand is to simplify the problem with suitable assumptions and choose the appropriate block of equations from Section 3.9. Simplification The system, obviously closed, is uniform so that a single state describes its state at a given time. The system is obviously unsteady, its image taken with a state camera changing with time. However, the problem description clearly indicates the system travels from a b-state to a f-state, the hallmark of any process. The block of equation summarized in Section 3.9.3, therefore, describes the appropriate form of the balance equations. The equations can be further simplified by noting that changes in KE and PE are most likely negligible making E U . Mass Energy m constant 0 0 E U KE PE Q W 0 Or, U m u f ub Q Entropy S S f Sb k Exergy Qk Sgen,univ Tk T 0 f b Qk 1 0 Wu T0 Sgen,univ k Tk Simplification Using TEST Starting at the Daemons page, progressively navigate through Closed, Process, Generic and Uniform pages. A system schematic and the set of equations that describe that system are displayed at the bottom of the page. An appropriate material model is selected as the last step before the Closed Process daemon is launched. Discussion The boundary temperature is unknown in this problem. Since the body is being heated to a temperature of 100 o C , at least one of the heat sources must be at a temperature of 100 o C or more. Also note that the MEEE equations derived in this problem are 3-34 Fig. 3.47 Heating the block from a b-state to a f-state constitutes a closed process. applicable regardless of the model chosen. Individual terms of the balance equations will be discussed in the next two chapters. Notice that the equations are derived here for the extended system. Also observe that the balance equations in their current form are independent of the material model. EXAMPLE 3-3 MEEE Equations for a Closed Process. Develop the appropriate form of MEEE (mass, energy, entropy and exergy) equations for the following problem. A piston-cylinder device initially contains 20 g of saturated water vapor at 300 kPa. A resistance heater is operated within the cylinder with a current of 0.4 A from a 240 V source until the volume doubles. At the same time a heat loss of 4 kJ occurs. Determine the final temperature and the duration of the process. SOLUTION To develop a customized set of MEEE equations. Simplification The simplification carried out in Ex. 3-2 applies to this problem as well. In addition to heat transfer, there are two modes of work transfer, electrical and boundary work. The closed process equations of Section 3.93 can be simplified as follows. Mass Energy m constant 0 0 E U KE PE Q W Q WB WO Or, U m u f ub Q WB WO Entropy S S f Sb k Exergy Qk Sgen,univ Tk T 0 f b Qk 1 0 Wu T0 Sgen,univ k Tk Simplification Using TEST The procedure remains unchanged to the one described in the last problem. Discussion Steam trapped in a piston-cylinder device apparently has no similarity with the block of aluminum of the last example. However, as far as the governing MEEE equations are concerned, the only difference between the two systems is the presence of work transfer in this problem. As in the previous problem, the balance equations in their current form are independent of the material model. EXAMPLE 3-4 MEEE Equations for a Non-Mixing Closed Process. 3-35 Fig. 3.48 Steam undergoes a closed process just like the block in Fig. 3.47. Develop the appropriate form of MEEE equations for the following problem. A 40 kg aluminum block at 100 o C is dropped into an insulated tank that contains 0.5 m3 of liquid water at 20 o C . Determine the entropy generated in this process. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification Water and the block constitute a non-uniform closed system going through a process in this problem. Two states, one for the block and one for water, can be used to describe the composite begin state. At the end of the process, even though the temperature is uniform, the finish-state still requires a composite description as the density is different for the two sub-systems. Designating the two subsystems as A and B, and neglecting any changes in KE and PE , the closed process equations can be simplified as follows. Mass Energy Fig. 3.49 The composite system goes through a non-mixing closed process. mA constant; mB constant; 0 0 0 E U KE PE Q W 0 Or, U U f U b mAu f , A mBu f , B mAub , A mBub , B 0 Entropy S S f Sb k 0 Qk Sgen,univ Tk Or, S S f Sb mA s f , A mB s f , B mA sb , A mB sb , B S gen 0 0 T f b Qk 1 0 Wu T0 Sgen k Tk Or, f b mA f , A mB f , B mAb , A mBb , B T0 Sgen,univ Exergy Simplification Using TEST Navigate through the Systems, Closed, Process, Generic, Non-Uniform, Non-Mixing, pages to display the progressively simplified system schematic and balance equations. Discussion The subsystems are closed themselves since there is no mass transfer between them. In TEST such systems are called nonmixing non-uniform systems. In the following example, on the other hand, the subsystems of a non-uniform system can be seen to be mixing. As in the previous problem, the balance equations in their current form are independent of the material model. EXAMPLE 3-5 MEEE Equations for a Mixing Closed Process. 3-36 Fig. 3.50 The composite closed system goes through a mixing process. Develop the appropriate form of MEEE equations for the following problem. A 0.5 m3 rigid tank containing hydrogen at 40 o C , 200 kPa is connected to another 1 m3 rigid tank containing hydrogen at 20 o C , 600 kPa. The valve is opened and the system is allowed to reach thermal equilibrium with the surroundings at 15 o C . Determine the irreversibility in this process. Assume variable c p . SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification By drawing the system boundary as shown in the accompanying figure, gases in the two tanks, each of which acts as an open system during the process, behave like a closed system. In the resulting non-uniform system, two states, one for tank A and one for tank B, must be used to describe the composite begin state. At the end of the mixing process, the finish state is uniform and can be represented by a single state. Neglecting any changes in KE and PE , the closed process equations can be simplified as follows. mA mB constant; Mass 0 0 Energy E U KE PE Q W Entropy S S f Sb 0 Or, U U f U b mA mB u f mAub , A mBub , B Q k Qk Sgen Tk S S f Sb mA mB s f mA sb , A mB sb , B Q S gen,univ T0 0 Exergy T 0 f b Qk 1 0 Wu T0 Sgen,univ k Tk f b mA mB f mAb , A mBb , B T0 Sgen,univ Simplification Using TEST Navigate through the Systems, Closed, Process, Generic, Non-Uniform, Mixing, pages to display the progressively simplified system schematic and balance equations. Discussion An interpretation of different terms of the balance equation is postponed until the next chapter. If the valve is closed before mixing is complete, the finish state must be expressed through a composite state just like the begin state. The balance equations, it should be noted, are independent of the material model. 3-37 EXAMPLE 3-6 MEEE Equations for a Closed Steady System. Develop the appropriate form of MEEE equations for the following problem. Fig. 3.51 A closed system at steady state. A10 m2 brick wall separates two chambers at 500 K and 300 K respectively. If the rate of heat transfer is 0.5 kW/m2, determine the entropy generation rate and the rate of exergy destruction in the wall. Assume the wall surface temperatures to be the same as the adjacent chamber temperatures. Also assume steady state. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification The brick wall in this problem, obviously, constitutes a closed system at steady state. Because the area of the wall at the edges are negligible compared to the two main faces, heat transfer through the end faces can be neglected. Also the time derivatives of KE and PE can be assumed zero. Mass m constant; 0 Energy 0 QH QC W ; QH QC Entropy 0 k Exergy Qk Sgen,univ Tk 1 1 Sgen,univ QH TC TH 0 T 0 Qk 1 0 Wu T0 Sgen,univ k Tk T T 0 QH 1 0 QC 1 0 T0 Sgen,univ TH TC Simplification Using TEST Navigate through the Systems, Closed, Steady pages to display the progressively simplified system schematic and balance equations . Discussion Once again we will defer interpretation of various terms until the next chapter. With QH QC , the exergy equation can be shown to reduce to entropy equation for this particular system. Notice that the equations are derived here for the extended system. EXAMPLE 3-7 MEEE Equations for an Open Steady System. Develop the appropriate form of MEEE equations for the following problem. Carbon dioxide enters steadily a nozzle at 35 psia, 1400 o F , and 250 ft/s and exits at 12 psia and 1200 o F . Assuming the nozzle to be 3-38 Fig. 3.52 A nozzle operating at steady state. adiabatic and the surroundings to be at 14.7 psia, 65 o F , determine (a) the exit velocity, and (b) the entropy generation rate by the device and the surroundings. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification The image of the nozzle taken with a state camera remains frozen even though the state of the fluid flowing through the nozzle changes. Hence, a nozzle is an open steady device. Although change in PE can be neglected, the purpose of a nozzle is to accelerate a flow and, therefore, the change in KE must be considered significant. Because there is a single flow through the nozzle, the summation over inlets and exits of the open, steady equations of section 3.9.5 reduce to Mass mi mi m Energy 0 m ji je Q Wext ; 0 0 ji je 0 0 m si se Entropy k Exergy se si Qk Sgen,univ m si se Sgen,univ Tk Sgen,univ m 0 0 T 0 m i e Qk 1 0 Wu T0 Sgen,univ k Tk TS e i 0 gen,univ m Simplification Using TEST Navigate through the Systems, Open, Steady, Generic, and Single-Flow pages to display the progressively simplified system schematic and balance equations. Discussion Individual terms of the balance equations will be discussed in the next two chapters. Notice that the equations are derived here for the extended system. Also observe that the balance equations in their current form are independent of the material model. EXAMPLE 3-8 MEEE Equations for a Mixing, Open Steady System. Develop the appropriate form of MEEE equations for the following problem. 3-39 Liquid water at 100 kPa and 10 o C is heated by mixing it with an unknown amount of steam at 100 kPa and 200 o C , and by heating the mixing chamber with a resistance heater with a power rating of 5 kW. Liquid water enters the chamber at 1 kg/s, and the chamber looses heat at a rate of 500 kJ/min with the ambient at 25 o C . If the mixture leaves at 100 kPa and 50 o C , determine (a) the mass flow rate of steam, and (b) the entropy generation rate during mixing. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification The mixing chamber can be assumed to operate at steady state. Although heat is transferred from the electrical heating elements to the working fluid, it is electrical power Wel that crosses the boundary and, therefore, must appear in the energy and exergy equations as Wext and Wext,u respectively. Two inlet states, i1-State and i2-State, and one exit state, e-state, are required in this multi flow mixing configuration. The open, steady equations of section 3.9.5 reduce to Mass mi1 mi 2 me Energy 0 mi1 ji1 mi 2 ji 2 me je Q Wel ; Entropy 0 mi1si1 mi 2 si 2 me se Q Sgen,univ T0 Exergy 0 T 0 mi1 i1 mi 2 i 2 me e Qk 1 0 Wu T0 Sgen,univ k Tk 0 mi1 i1 mi 2 i 2 me e Wel T0 Sgen,univ Simplification Using TEST Navigate through the Systems, Open, Steady, Generic, Multi-Flow-Mixed pages to display the progressively simplified system schematic and balance equations . Discussion Individual terms of the balance equations will be discussed in the next two chapters. Notice that the equations are derived here for the extended system. Also observe that the balance equations in their current form are independent of the material model. EXAMPLE 3-9 MEEE Equations for a Non-Mixing, Open, Steady System. Develop the appropriate form of MEEE equations for the following problem. 3-40 Fig. 3.53 A steady state mixing chamber. Steam enters a closed feedwater heater at 1.1 MPa and 200 o C and leaves as saturated liquid at the same pressure. Feedwater enters the heater at 2.5 MPa and 50 o C and leaves 12 o C below the exit temperature of steam. Neglecting any heat losses, determine (a) the mass flow rate ratio and (b) the entropy generation rate of the device and its surroundings. Assume surroundings to be at 20 o C . Fig. 3.54 A closed feed water heater used in a steam power plant. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification The closed feed water heater shown in the accompanying figure is a heat exchanger, where the flow of water is heated by the flow of steam. For this non-mixing multi-flow configuration, two inlet states, i1- and i2-states, and two exit states, e1- and e2-states, describe the two flows, flow-A from i1 to e1 and flow B from i2 to e2. Clearly there is no external work transfer for this passive device. The open, steady equations of section 3.9.5 simplify into Mass mi1 me1 mA ; mi 2 me 2 mB ; Energy 0 mi1 ji1 mi 2 ji 2 me1 je1 me 2 je 2 Q Wext ; 0 0 mA ji1 je1 mB je 2 ji 2 0 Entropy 0 mi1si1 mi 2 si 2 me1se1 me 2 se 2 Q TB Sgen,univ 0 mA si1 se1 mB si 2 se 2 Sgen,univ 0 mi1 i1 mi 2 i 2 me1 e1 me 2 e 2 Exergy 0 0 T Qk 1 0 Wu T0 Sgen,univ k Tk 0 mA i1 e1 mB i 2 e 2 T0 Sgen,univ Simplification Using TEST Navigate through the Systems, Open, Steady, Generic, Multi-Flow Non-Mixing pages to display the progressively simplified system schematic and balance equations . Discussion Individual terms of the balance equations will be discussed in the next two chapters. EXAMPLE 3-10 MEEE Equations for an Open Process. Develop the appropriate form of MEEE equations for the following problem. 3-41 Fig. 3.55 The selection of the inlet state on the outer side of the valve ensures that State-i remains unchanged during the open process. An insulated rigid tank is initially evacuated. A valve is opened, and air at 100 kPa 20 o C enters the tank until the pressure in the tank reaches 100 kPa when the valve is closed. Determine the final temperature of the air in the tank. Assume variable specific heats. SOLUTION To simplify the problem so that the balance equations can be reduced to one of the customized forms discussed in this chapter. Simplification The tank, an open system, goes from a vacuum bstate to a filled f-state as air from the supply line rushes in. If the istate is located above the position of the valve, its thermodynamic state at all times can be considered identical to that in the supply line. In this open-process , there is no external work or heat transfer. The open, process equations of section 2.9.5 simplify into 0 m m f mb mi ; Or, m f mi Mass Energy 0 0 0 E f Eb mi ji Q Wext ; 0 m f u f ke pe u f hi 0 m h ke i i 0 pe 0 Entropy Q 0 S f Sb mi si 0 TB Sgen,univ m f s f mi si Sgen,univ s f si Sgen,univ mf Exergy 0 0 T f b mi i Qk 1 0 k Tk m f f mi i T0 Sgen,univ f i 0 Wu T0 Sgen,univ T0 Sgen,univ mf Simplification Using TEST Navigate through the Systems, Open, Process pages to display the progressively simplified system schematic and balance equations. Discussion Individual terms of the balance equations will be discussed in the next two chapters. 3-42 3.10 Summary The fundamental governing equations for the interactions between a system and its surroundings are derived in a common format called the balance equation in this chapter. The goal is to express the governing equations in a customized format for a given system. The Reynolds transport equation or the RTE relates the rate of change of any total extensive property of an open system at a given instant with that of a closed system passing through, which happens to occupy the entire open system at that time. With the help of RTE the fundamental laws of thermodynamics, postulated for a closed system, are converted into balance equation for a very general system. In Section 3.3 systems are classified into a tree structure with different branches representing groups of systems that show some similar patterns. Mass balance equation is derived and expressed in different formats in Section 3.4. Similarly, energy, entropy, exergy, and momentum equations are derived in Sections 3.5 through 3.8. Finally, in Section 3.9 the complete set of equations, called the MEEE equations are summarized for important classes of systems that are often encountered in the practice of thermodynamics. The next two chapters are devoted to understanding the various equations derived in this chapter through comprehensive analysis of various closed and open systems. 3.11 Index anchor states, 3-9 atmospheric work, 3-22 axioms, 3-1 balance equation, 3-4 Balance Equation, 3-3 Balance Equations Closed Process Form Summary, 3-31 Closed Steady Form Summary, 3-32 Open Process Form Summary, 3-33 Open Steady Form Summary, 3-32 Balance Equations, Closed Systems Summary, 3-30 Balance Equations, General Form Summary, 3-30 begin-state, 3-9 Classification of Systems, 3-6 Closed Systems, 3-7 conservative form, 3-12 dead state, 3-23 Energy Balance Different Forms, 3-14, 319 Entropy Balance Equation, 3-15 exergy, 3-20, 3-24 Exergy Balance Different Forms, 3-26 Exergy Balance Equation, 320 exergy destruction, 3-25 extended system, 3-17 final-state, 3-9 First Law, 3-11 flow diagram, 3-10 general balance equation, 3-6 3-43 general balance equation, energy, 3-13 general balance equation, entropy, 3-18 general balance equation, exergy, 3-24 general balance equation, momentum, 3-29 generalized friction, 3-16 heat reservoir, 3-21 irreversibility, 3-17, 3-25 irreversible, 3-17 Mass Balance Different Forms, 3-11 mass balance equation, 3-10 Mass Balance Equation, 310, 3-11 MEEE equations, 3-29 mixing systems, 3-36 Momentum Balance Equation, 3-28 multi flow, 3-40 multi flow, non-mixing, 3-41 Newton’s Second Law, 3-28 non-mixing systems, 3-36 non-uniform systems, 3-36 open process, 3-9, 3-42 Open Systems, 3-7 process, 3-9 reversible, 3-17 Reynolds Transport Theorem, 3-5 RTE, 3-5 Second Law, 3-15 single flow, 3-39 specific flow energy, 3-13 steady flow energy equation, 3-15 steady state, 3-7 Steady Systems, 3-7 System classification, 3-10 System tree, 3-10 TER, 3-21 thermal energy reservoir, 3-21 uniform and steady flow, 3-9 unsteady, 3-7 Unsteady Instantaneous, 3-8 Unsteady Process, 3-8 Unsteady Systems, 3-7 3-44