Using Spectrophotometry to Determine Concentration
advertisement
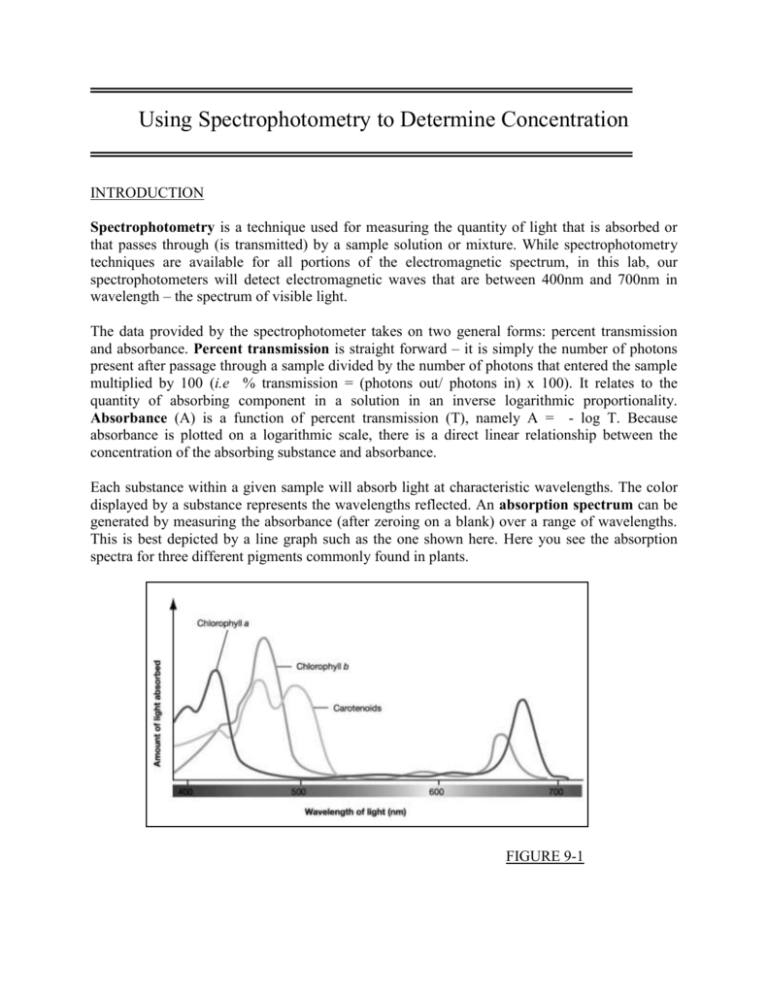
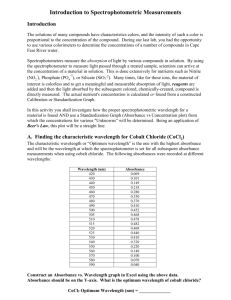
Using Spectrophotometry to Determine Concentration INTRODUCTION Spectrophotometry is a technique used for measuring the quantity of light that is absorbed or that passes through (is transmitted) by a sample solution or mixture. While spectrophotometry techniques are available for all portions of the electromagnetic spectrum, in this lab, our spectrophotometers will detect electromagnetic waves that are between 400nm and 700nm in wavelength – the spectrum of visible light. The data provided by the spectrophotometer takes on two general forms: percent transmission and absorbance. Percent transmission is straight forward – it is simply the number of photons present after passage through a sample divided by the number of photons that entered the sample multiplied by 100 (i.e % transmission = (photons out/ photons in) x 100). It relates to the quantity of absorbing component in a solution in an inverse logarithmic proportionality. Absorbance (A) is a function of percent transmission (T), namely A = - log T. Because absorbance is plotted on a logarithmic scale, there is a direct linear relationship between the concentration of the absorbing substance and absorbance. Each substance within a given sample will absorb light at characteristic wavelengths. The color displayed by a substance represents the wavelengths reflected. An absorption spectrum can be generated by measuring the absorbance (after zeroing on a blank) over a range of wavelengths. This is best depicted by a line graph such as the one shown here. Here you see the absorption spectra for three different pigments commonly found in plants. FIGURE 9-1 By measuring the absorption spectrum of a substance (i.e. all the wavelengths at which it absorbs) it is possible to identify it or at least place it in a particular class of compounds. The wavelength at which peak absorption occurs, the absorption maximum (λ max), is very useful when trying to identify an unknown. By creating and measuring a series of standards (e.g. serial dilutions), it is possible to quantify the amount or concentration of a substance in a sample. For example, in their pure form, the nucleic acids can be quantified by absorption measurements in the UV range. You will practice generating an absorption spectrum for food color dye using a Spec 20 spectrophotometer in Part I of this lab. Part II of this lab allows you to apply spectrophotometry in order to measure chlorophyll concentrations in olive oil. Olive oil is made by pressing/extracting the rich oil from the olive fruit by various methods. There are various grades of olive oil; three common grades are: extra virgin, regular, and light. Extra virgin olive oil is considered the highest quality. It is the first pressing from freshly prepared olives. It has a greenish-yellow tint and a distinctively fruity aroma because of the high levels of chlorophyll and other volatile materials extracted from the fruit. Regular olive oil is collected with the help of a warm water slurry to increase yield. It is pale yellow in color, with a slight aroma, because it contains fewer volatile compounds. Light olive oil is very light in color and has no aroma because it has been processed under pressure to remove the chlorophyll and volatile compounds. Light olive oil is commonly used for frying because it does not affect the taste of fried foods and it is relatively inexpensive. The absorbance spectrum, in the visible light range, of chlorophyll gives interesting results. The chemistry of chlorophyll creates three blue absorbance peaks at 416, 454, and 482 nm, and one red absorbance peak at 670 nm. That means that chlorophyll absorbs blue and red light well and transmits or reflects green light. This is why we perceive chlorophyll and the plant leaves that contain it to be various shades of green. Plants tend to absorb blue and red light well – green light, not so well. The primary objective of this experiment is to determine the concentration of various chlorophyll containing olive oils using their absorbance properties. You will use a Vernier Spectrometer to measure the absorbance by chlorophyll in each oil sample. You will measure the absorbance of extra virgin olive oil over the visible light spectrum and select the wavelength of maximum absorbance (λ max). A higher concentration of the solution absorbs more light (and transmits less) than a solution of lower concentration. The extra virgin olive oil has a chlorophyll concentration (molarity) of 9.82 x 10-6 mol/L. You will first calculate the chlorophyll concentration for each of the five serial dilutions that were made from the extra virgin olive oil. You will then use the Vernier spectrometer to measure the absorbance and transmittance of each dilution at the λ max. The graph of absorbance or transmittance vs. concentration for the serial dilutions will describe a direct relationship, known as Beer’s Law. Transmittance (T) is defined as the fraction of incident light which is transmitted (i.e. passes through) a sample. Thus, T = I/Io, where Io equals the intensity of light which strikes the sample and I is the intensity of light after passing through the sample. Transmittance is usually expressed as a percentage: %T = (I/Io) x 100 Absorbance (A), or optical density, is a logarithmic function of T and is expressed as: A = log10 (1/T) = log10 (Io/I) Note that absorbance has no units. The shorthand for absorbance is Axxx, where wavelength at which the measurement was made (e.g. A420). xxx is the So, for example, at 100% transmittance, A = log 1.0 = 0. At 50% transmittance, A = log (1/0.5) = 0.30. The Spec 20 spectrophotometer has two scales, one calibrated from 0 to 100% Transmittance and the other as Absorbance, ranging from infinity to 0. Note that the highest calibrated unit of absorbance is 2.0. Spectral data are usually plotted as absorbance (y-axis) versus wavelength or concentration (x-axis). The relationship between absorbance and transmittance is illustrated in the following diagram: FIGURE 9-2 So, if all the light passes through a solution without any absorption, then absorbance is zero, and percent transmittance is 100%. If all the light is absorbed, then percent transmittance is zero, and absorption is infinite. Notice that absorbance is basically a log-transformed version of transmittance which means that a plot of absorbance against concentration should result in a linear relationship. We will test this by plotting absorbance and transmittance on both regular graph paper and semi-log graph paper. You will determine the concentration of chlorophyll in lesser grades of olive oil by measuring their absorbance with a spectrometer and using the best-fit line equation of the Beer’s law curve to calculate the lesser grade oil’s chlorophyll concentrations. MATERIALS Vernier Spectrometer Vernier Logger Pro 3 software Spec 20 spectrophotometer extra virgin, regular, and light olive oil extra virgin olive oil serial dilutions (5) regular graph paper computer food dye solution cuvettes plastic pipettes semi-log graph paper PROCEDURE Using a Spec 20 The wavelength on a Spec 20 is selected by adjusting a prism within the instrument such that only a narrow range of light wavelengths are directed through the sample. In addition, the bulbs used to generate the light come in a variety of wavelength ranges. For general purpose work, we utilize broad range bulbs which allow absorbance to be measured over the entire visible light range. In order to measure the absorbance of a particular substance in a reaction mixture, it is necessary to first "zero out" the spectrophotometer such that only the absorbance of the substance of interest is measured. This is done with a blank - a cuvette which contains the carrier solvent without the substance of interest. Zeroing the Spec 20 1. Turn on the Spec 20 and allow it to warm up for 5 - 10 minutes (left front knob). Set wavelength using the dial on top of the Spec 20. FIGURE 9-3 2. Prepare a blank cuvette by adding the solvent (water) without the absorbing substance (the food dye). The blank is used to calibrate the Spec 20 so that any absorbance attributable to the solvent and/or glass cuvette can be compensated. By zeroing the Spec 20 to the blank, you will measure only the absorbance due to the substance in question. 3. With no tube in the holder, adjust the meter needle to read infinite absorbance (= 0% transmittance) using the left front knob. 4. Using a Kimwipe, wipe off/polish the outside of the blank cuvette to remove greasy finger smudges. Using a wax pencil or Sharpie, make a small vertical mark at the top of each cuvette for alignment in the sample holder. FIGURE 9-4 5. Raise the sample holder trapdoor and insert the cuvette such that the line on the cuvette lines up with the line on the sample holder. Close the lid. FIGURE 9-5 6. Using the right front knob, adjust the meter needle to read absorbance = 0.0 (= 100 % transmittance). This step is called setting the "full scale". FIGURE 9-6 The spectrophotometer is now calibrated to this blank at this wavelength. Measuring Absorbance on the Spec 20 7. Remove the blank and insert the cuvette containing the food dye solution. Close the lid. 8. Read the absorbance (lower scale) or transmittance (upper scale) as appropriate for your sample. Since you are creating an absorption spectrum, you should note the absorbance at each wavelength. FIGURE 9-7 9. Create an absorption spectrum for the food dye solution. Measure the absorbance from 400 to 700 nm at 10nm intervals. Remember that you must re-zero the Spec 20 with the blank every time you change the wavelength. Enter your absorbance values in the table provided here. Food Dye Solution Absorbance Values ג Absorbance ג Absorbance ג 400 500 600 410 510 610 420 520 620 430 530 630 440 540 640 450 550 650 460 560 660 470 570 670 480 580 680 490 590 690 Absorbance 700 TABLE 9-1 10. Graph the absorbance on the y axis against wavelength on the x axis to create an absorption spectrum for the food dye solution. At the end of this lab, check the absorption spectrum of the food dye solution using the Vernier spectrometer. Is the spectrum similar to the one you produced with the Spec 20? Using a Vernier Spectrometer to apply Beer’s Law 1. Obtain a rack with the following prepared cuvettes: extra virgin olive oil (known chlorophyll concentration) five serial dilutions of extra virgin olive oil (calculated chlorophyll concentration) regular olive oil (unknown chlorophyll concentration) light olive oil (unknown chlorophyll concentration) vegetable oil (used for dilutions – blank) 2. Calculate the chlorophyll concentration of the serial dilutions and enter those values in the results table. Each dilution is a 1:1 dilution, so the chlorophyll concentration is halved from each dilution to the next. Remember that the chlorophyll concentration in the extra virgin olive oil is 9.82 x 10-6 mol/L. 3. Start the LabQuest Logger Pro 3 program. 4. Calibrate the spectrometer. a) Use the cuvette with vegetable oil as the blank. Be careful not to touch the sides of the cuvette so as not to get fingerprints on the surface. Place the blank cuvette in the spectrometer. Align the cuvette so that the clear sides are facing the light source of the spectrometer. b) Select Calibrate ► Spectrometer from the Experiment menu. The calibration dialog box will display the message: “Waiting.......seconds for lamp to warm up”. The minimum warm up time is one minute. Follow the instructions in the dialog box to complete the calibration. Click 5. Determine the wavelength of maximum absorption (ג olive oil. for chlorophyll a in extra virgin a) Take the cuvette with the extra virgin olive oil and place it in the spectrometer. b) Click A full spectrum graph of the oil will be displayed. Note that one area of the graph contains a peak absorbance (λ max). Click to complete the analysis. Record the wavelength of maximum absorbance (e.g. 416). 6. Record all of the absorbance values for each serial dilution of the extra virgin olive oil, the regular olive oil, and light olive oil at גmax. 7. To obtain transmittance values go to ‘Experiment’ to ‘change units’. Select ‘% transmittance’. Record transmittance values for each serial dilution and the regular olive oil and light olive oil at גmax. Solution extra virgin olive oil (EVOO) serial dilution 1 (EVOO) serial dilution 2 (EVOO) serial dilution 3 (EVOO) serial dilution 4 (EVOO) serial dilution 5 (EVOO) regular olive oil (unknown 1) light olive oil (unknown 2) Concentration (mol/L) x 10-6 Absorbance at גmax Transmittance at גmax 9.82 TABLE 9-2 8. Graph absorbance and transmittance against concentration. Apply Beer’s Law to estimate the concentrations of the regular and light olive oil. Enter those values in the table. In addition to the table, also include graphs showing the data and linear-regression equation for the serial dilutions. Your graphs are easy to create in Excel. Excel will automatically calculate the linear-regression equation for you. DISCUSSION QUESTIONS 1) What is the relationship between percent transmission and absorbance? 2) Why is absorbance data most frequently used to determine the concentration of a substance in a solution? 3) If an unknown substance has a green color, what wavelengths would you expect that it does not absorb? 4) How did you use the standard curve that you generated for the serial dilutions to determine the quantity of chlorophyll a in the two lesser grades of olive oil? ADAPTED FROM: Spectroscopy with Vernier © 2006 Vernier Software & Technology