Physical Structures: Capsids, Envelopes & Nucleocapsids
advertisement

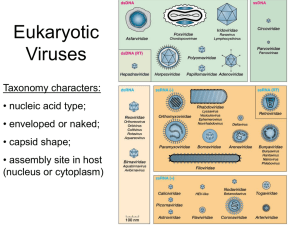
BSCI 437: Lecture 3. Physical Structures: Capsids, Envelopes & Nucleocapsids. Great web resource: VIPER virus particle explorer. http://mmtsb.scripps.edu/viper/ Capsids: Composed of numerous repeating subunits – identical or belonging to only a few different species – arranged in precisely defined patterns. This strategy 1) minimizes the amount of genetic information required to specify capsids, and 2) assures efficient assembly. Capsids both 1) protect viral genomes and 2) help to introduce viral genomes into host cells. Envelopes: Most animal viruses are enclosed by membrane-containing envelopes that are acquired as the nucleocapsids bud through special patches of cell membranes on their way out of the cell. Envelopes usually only contain virus-specified proteins and glycoprotein spikes. The lack of rigidity makes enveloped viruses appear heterogeneous in shape and size when fixed for EM. However, in their native state, most enveloped viruses are spherical: exceptions are the Rhabdoviruses (bulletshaped), and Influenza virus (long and filamentous due to the shape of the underlying nucleocapsid. Nucleocapsids: Two basic structural patterns: helical and icosahedral. Helical: The topology of the nucleocapsid follows the biophysical geometry of the nucleic acid genome. Similar to chromatin. Examples include Tobacco Mosaic virus, Influenza, and HIV. Icosahedral: the nucleocapsid follows the constraints of Euclidean solid geometry. This geometry provides the most efficient way to enclose a maximum volume, and as such, viral genomes are condensed inside of them. The icosahedral sphere has 5-, 3and 2-fold axes of symmetry. The basic unit is 60 multiplied by a “symmetry number”. Most viruses use this strategy. Size, mass, dimensions of viruses (show pictures of several virus types) Particle- An aggregate of many molecules often of different nature. Virus particles have particle weights ranging form 3-800 X 106 Daltons (Da). Particle (Virion) linear dimensions are generally given in nm (10-9 meters) with typical viruses ranging from about 25-several hundred nm across. Bacterial cells are about 1500 nm and Eukaryotic epithelial cells about 20,000 nM. Assuming viruses and bacteria are nearly spherical (not always the case), a bacteria has a volume about 30,000 times greater than a virus while a epithelial cell is about 60 million times larger. This means we could stuff about 3 x 1014 virus particles in one drop of water. S-value- A value derived from the sedimentation rate of particles and molecules in the ultracentrifuge. Numbers are reproducible under specific conditions and reflect the volume and shape of a particular substance but are not directly proportional to mass. The basic unit is the Svedberg (S) which is 10-13 sec. This value can be used to estimate molecular weights in conjunction with other values. Structural Principles and terms: Helical-Rod or threadlike appearance, ie. Rhabdoviruses (rabies virus), Tobacco Mosaic 1 virus. Isometric-spherical appearance, ie. Picornaviruses (poliovirus) Irregular-without clear symmetry, ie. Poxvirus (smallpox) Virus makeup: protein subunit-individual folded protein molecules. structural subunit- (synonyms; protomer, asymmetric unit)- Unit from which capsid of nucleocapsid are built; may comprise one protein subunit or multiple different subunits. morphological unit (syn: capsomere)- surface structure (knobs, projections, clusters, etc.) seen by electron microscopy. This term is generally restricted to descriptions of viruses from electron micrographs. capsid (syn: coat)- regular, shell-like structure composed of aggregated protein subunits which surrounds the viral nucleic acid. nucleocapsid (syn: core)- viral nucleic acid enclosed by a capsid protein coat. envelope (syn: viral membrane)- lipid bylayer containing viral glycoproteins. The phospholipids in the bylayer are derived from the cell that the virus arose from. Not all viruses have envelopes some consist of only the nucleocapsid. virion- physical virus particle. Nucleocapsid alone for some viruses (picornaviruses) or including outer envelope structure for others (retroviruses). Viral Capsid Structure, Assembly, and interactions with Receptors Capsid- Protein coat that encapsidates the viral genome. Nucleocapsid-Capsid with genome inside (plus anything else that may be inside like enzymes and other viral proteins for some viruses). Capsid functions: 1. Protect genome from atmosphere (May include damaging UV-light, shearing forces, nucleases either leaked or secreted by cells). 2. Virus-attachment protein- interacts with cellular receptor to initiate infection. Since viruses are made of many different repeated subunits thereis redundancy; Many receptor sites so damage to a few doesn’t prevent infection. 3. Delivery of genome in infectious form. May simply “dump” genome into cytoplasm (most +ssRNA viruses) or serve as the core for replication (retroviruses and rotaviruses). How do particles form? The information for formation is encoded in the components (nucleic acid + proteins). Some proteins can form capsid shells in the absence of the genome while others form around the genome. In 1955 Fraenkel-Conrat and Williams showed that TMV nucleocapsids would form spontaneously from individual protein subunits (coat protein) and the genome. Therefore particles represent a free energy minimum state which leads to stability. Assembly is driven by hydrophobic and hydrophilic (rarely covalent) interactions. They are protein-protien, protein-nucleic acid, and protein-lipid interactions. Note- particles must disassemble at some point during infections, covalent bonds would make this more difficult. Why not make the capsid from a single large protein rather than assemble it from 2 many proteins? There is not enough genomic information: MW of 1 codon (3 nucleotide) is about 1000----1 amino acid is about 150, a genome can only produce proteins that are 15% of its molecular weight. A picornavirus is about 10,000 bases----can produce a protein about 500,000 Dal. Outer shell of picornaviruses is make up of 60 copies each of 4 different proteins, approx MW=2 million. The genome does not have enough information to encode a single protein that could encapsidate it. Virus Shapes Most viruses have evolved to form one of 2 different shapes; Helical or icosohedral. Some irregular viruses do exits and many of these have underlying helical or icosohedral symmetry. Note-viruses form regular shapes but use irregular proteins to do so. This creates a problem that must be solved for assembly to occur. For example, it would be easy to imagine how a virus might form an icosahedron if perfectly triangular proteins were used. But the proteins are irregular shaped and still must form a sealed (to protect genome) icosahedron. Helical Viruses Many biological components have adopted a helical structure (DNA, helix of proteins). A helix is energetically favorable and can allow flexibility (bend but don’t break (shear)). The simplest way to arrange irregular identical proteins would be around a central axis to form a disk. Disks could then be stacked with the genome in the middle to form a cylinder. Helical viruses form a closely related spring like helix instead. The best studied TMV but many animal viruses and phage use this general arrangement. Note-all animal viruses that are helical are enveloped, unlike many of the phage and plant viruses. Most helixes are formed by a single major protein arranged with a constant relationship to each other (amplitude and pitch). They can be described by their Pitch (P, in nm): P= x p, -# of protein subunits per helical turn, p-axial rise per subunit VSV, a prototypical helical animal virus (See fig 4.4) Properties of a “typical” helical coat protein. The protein is small (50 aa derived from a 73 aa precursor), helical with 3 distinct domains; a positively charged COOH terminus oriented toward the genome, a hydrophobic central domain, and a negatively charged outward facing NH2 terminus. The domain characteristics are consistent with their function, + charge interacts with nucleic acid, hydrophobic with proteins on either side, negative charge with polar environment. Note that each subunit is tilted 20o relative to the long axis of the particle. P= 6.75 nm, =4.5, and p=1.5. Genome: 11,000 base -ssRNA interacts with the nucleocapsid protein (N) to form a helical structure with P=5 nm. The particle is about 180 nm long and 80 nm wide. N is 50 kDal, arginine and lysine rich, 9 x 5 x 3 nm and covers 9 nucleotides. It interacts with the genome on one side and the matrix (made of matrix (M) protein) on the other. The 3 matrix then interacts with the envelope and envelope proteins which cover the nucleocapsid. Most enveloped viruses have a matrix structure between the nucleocapsid and envelope. Many viruses have N proteins, general functions include: 1. Stabilize charge of genomic material (along with ions (K+ for example) that are often packaged in virion. 2. Protect from chemical, physical, enzymatic damage. 3. Sometimes they are required for replication (VSV, HIV). Icosahedral Viruses Structure with 12 vertices and 20 equivalent faces. It has a 2-fold, 3-fold, and 5-fold axis of symmetry. Viruses with this general shape are composed of 60 or more subunit proteins (generally more) that make up the outer surface. They are characterized by a Triangulation number (T): T=f2 x P where f=# of subdivisions on each side of a triangular face, P=h2 + hk + k2 where h and k are any nonnegative integer. In practice, T is the number of smaller identical equilateral triangles within each face. Only T’s that may be derived from the above equation are possible. With 60 being the minimal number of irregular subunits required (You might imagine 20 but they would have to be perfect triangles which proteins do not form) to make an icosahedron, we can make an equivalent structure, each protein can have identical interactions with its neighbor. Beyond 60 subunits equivalence is not possible. With 240 identical subunits and a T=4 structure we get pentamers and hexamers, so bonds between individual proteins cannot be equivalent. We must invoke quasi-equivalence- subunits in nearly the same local environment form nearly equivalent bonds with their neighbors. Viruses with T=1, 3, 4, 9, 16, and 25 have been found thus far. Picornaviridae, a prototype T=3 virus Fig. 4.9 illustrates quasi-equivalence with pentamer at each vertex and hexamers in other regions; Triangulation # = 3. Note that VP-4 is not on the surface of the structure but lies under the face. The protein subunits that form each protomer all assume a similar (not identical) shape (Fig. 4.9B). In fact all T=3 RNA viruses have proteins that form “8 strand antiparallel barrels”. The structures form from the polypeptide by first forming a “jelly-roll barrel” that then goes on to form the wedge-shaped barrel when the capsid is being formed. Complex structures. There are many viruses that are so large that they have evolved complex, more “cell like” structures. • e.g. Poxviridae, paramyxoviridae, Coronaviridae) How do capsids interact with the genomic RNA rather than other cellular RNA? -In many cases viruses produce huge amounts of viral genome and inhibit cell mRNA synthesis (either directly or indirectly). This helps but does not solve the problem. 4 -Viral genomes have packaging signals (“psi”) that form structure recognized by one of the capsid proteins. Keep in mind that viruses never do anything correct 100% of the time (defective particles). Where do virus capsids form? -For nonenveloped viruses they generally form in the cytoplasm (some in the nucleus) and mature before being released when the cell lyses. -Enveloped Viruses. The viruses generally form the capsid as they are budding from the envelope. Envelope proteins are inserted into the host cell membrane prior to budding. Viral matrix and capsid proteins interact with the membrane and membrane proteins at regions with a lot of envelope proteins. The genome interacts with the nucleocapsid protein and budding is initiated. See http://home.ncifcrf.gov/hivdrp/Freed_figure.html 5