File
advertisement

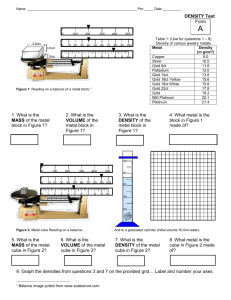
DENSITY measurements Name____________________________________________ Objectives measure mass with an electronic scale (or triple beam balance) measure volume using a graduated cylinder determine the density of different substances from mass and volume measurements calculate the percentage error in your results Background In order to measure a density, you need to take two measurements: 1. Mass, in grams, using an electronic scale. All masses measured to hundredths place. 2. Volume, in milliliters, using a graduated cylinder. All volumes measured to tenths place or ones place, depending on the size of the cylinder. NOTE: A milliliter (mL) is the same as a cubic centimeter (cm3) which is also known as a cc. Once you have these two measurements, divide them to determine a density! Use the equation: D m V Notes: Measure water volume at the bottom of the meniscus. Use the same balance for the entire lab (do not move the balance!) Remember to read volumes to tenths place and masses to hundredths place Procedure for solids: Here you directly weigh the piece of metal, and then obtain the volume of the metal by water displacement and subtraction. 1. Choose an unknown metal, weigh a piece (or pieces if very small) and record the mass. (Note: the piece or pieces should fit into your graduated cylinder) 2. Fill the graduated cylinder about ½ full. Record the water volume 3. Tilt the graduated cylinder and gently slide the metal piece(s) down inside it. Record the new water volume. Do not splash! 4. Calculate the volume of the metal and record. (subtract) 5. Calculate the density of the metal and record. (use density formula and divide). Density values should be to hundredths place. 6. Repeat steps 1-5 for each unknown metal. Data & calculations for solids: Metal A 1. Mass of metal Metal B Show calculations here! Metal A: 2. Volume of water (starting) 3. Volume of water + metal 4. Volume of metal [calculate by subtracting step 2 from step 3] 5. Experimental density of metal [calculate using D= m/V. Show work at right] 6. Most likely identity of metal Metal B: [see table pages 971-973] NOTE: metals are black on periodic table hanging up in the room and yours CANNOT be in 1st or 2nd column. 7. Theoretical density of metal [from pages 971 to 973] 1 Procedure for liquids: Here you get the mass of the liquid by first getting the mass of the cylinder and then subtracting the weight of the cylinder from the combined mass, but you can measure the volume directly in the cylinder. 1. Get any size graduated cylinder and mass it. Record in the table 2. Put about half full of water in it and mass it again. Record in the table 3. Calculate the mass of the liquid by subtracting the two values. 4. Read the graduated cylinder at the meniscus and record the volume to the nearest 0.5 ml. (that is, to the tenths place in intervals of 0.5) 5. Calculate density Data & calculations for liquids: water methanol Show calculations here! 1. Mass of cylinder Water: 2. Mass of cylinder + liquid 3. Mass of liquid 4. Volume of liquid [read the cylinder to the tenths place] 5. Experimental density of liquid [calculate using D= m/V. Show work at right] 6. Theoretical density Methanol: 1.00 g/mL 0.792 g/mL 1. Calculate the % error for all four measurements. Formula is: % error exp erimental value theoretical value theoretical value x 100 Use the your experimentally calculated density and the theoretical density values from the book and show all work. Metal A: Metal B: Water: Methanol: 2 2. Today’s method to find density would not work for all solids and liquids. State one reason why it might not work for all solids and one reason it would not work for all liquids. 3. “Error analysis”. You aren’t expected to be perfect in high school or anywhere. Suggest 3 very specific reasons that your calculated densities may not be accurate. These should include only lab errors, not calculation mistakes. You’ll need to think of how you did the lab and anything that you or your partner(s) did that could have been better. Or you can think of how to make the actual lab better. Again, be specific. 4. (Find answer online; write a paragraph here. DO NOT print off an entire article!) Archimedes was famous for yelling “Eureka” at being able to determine if the king’s crown was really pure gold. In fact, he was so excited at figuring out how to do this that legend has it he jumped out of his bath and ran through the streets naked yelling Eureka! Explain how he was able to use the concept of density to determine if the crown was really pure gold or a cheaper alloy of gold with silver. (by the way – he found it to be the cheaper version!). Go online and “google” Archimedes. Explain is some detail – like you were explaining it to a 5th grader, not me! 3 PRE-LAB QUESTIONS – DENSITY (Read the introduction on the front of lab paper) NAME(S)_________________________ 1. How is mass measured? 2. How is volume measured? 3. What unit(s) measures mass? 4. What unit(s) measures volume? 5. What unit(s) measure density? 6. What other units can be used that mean the same thing as milliliter? 7. a) What is the mathematical formula for density? b) Use algebra to solve for mass. c) Use algebra to solve for volume. 8. Which is heavier – a pound of lead or a pound of feathers? Why? 9. Which is heavier – a gallon of lead or a gallon of feathers? Why? 10. Show work to calculate the density of mercury if 11.1 cm3 of it had a mass of 149 grams. Show units in all of your calculations. Round answer to three decimal places. Would mercury sink or float in water? 11. Show work to calculate the mass of 15.1 mL of lead if the density of lead is 11.342 g/mL. Show units in all of your calculations. Round to two decimal places. Would lead sink or float in water? 12. Show work to calculate the volume of 255.g of a liquid with a density of 0.897 g/cm3. Round to two decimal places. Does this liquid sink or float in water? 4 Write the correct volume for each: 5