Atomic Collisions - Jordan University of Science and Technology
advertisement
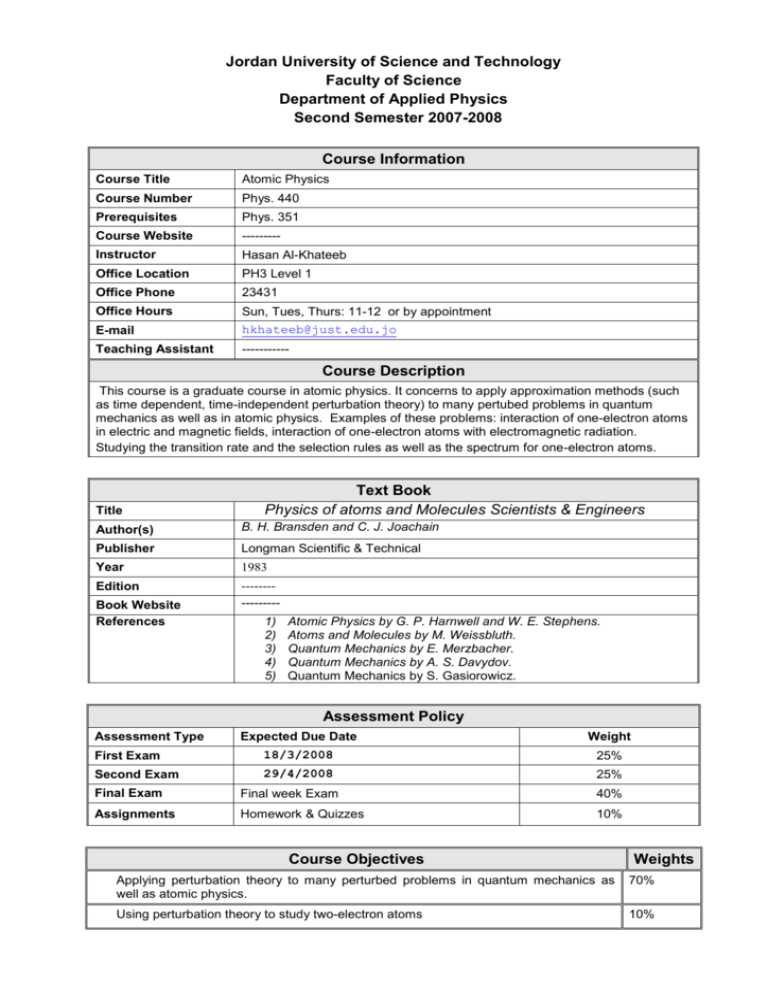
Jordan University of Science and Technology Faculty of Science Department of Applied Physics Second Semester 2007-2008 Course Information Course Title Atomic Physics Course Number Phys. 440 Prerequisites Phys. 351 Course Website --------- Instructor Hasan Al-Khateeb Office Location PH3 Level 1 Office Phone 23431 Office Hours Sun, Tues, Thurs: 11-12 or by appointment E-mail hkhateeb@just.edu.jo Teaching Assistant ----------- Course Description This course is a graduate course in atomic physics. It concerns to apply approximation methods (such as time dependent, time-independent perturbation theory) to many pertubed problems in quantum mechanics as well as in atomic physics. Examples of these problems: interaction of one-electron atoms in electric and magnetic fields, interaction of one-electron atoms with electromagnetic radiation. Studying the transition rate and the selection rules as well as the spectrum for one-electron atoms. Title Text Book Physics of atoms and Molecules Scientists & Engineers Author(s) B. H. Bransden and C. J. Joachain Publisher Longman Scientific & Technical Year 1983 Edition ---------------- Book Website References 1) 2) 3) 4) 5) Atomic Physics by G. P. Harnwell and W. E. Stephens. Atoms and Molecules by M. Weissbluth. Quantum Mechanics by E. Merzbacher. Quantum Mechanics by A. S. Davydov. Quantum Mechanics by S. Gasiorowicz. Assessment Policy Assessment Type Expected Due Date Weight First Exam 18/3/2008 25% Second Exam 29/4/2008 25% Final Exam Final week Exam 40% Assignments Homework & Quizzes 10% Course Objectives Weights Applying perturbation theory to many perturbed problems in quantum mechanics as well as atomic physics. 70% Using perturbation theory to study two-electron atoms 10% Studying many-electrons atoms 10% Studying the atomic collisions 10% Useful Resources Course Content Week 1 Topics Chapter in Text (sections) Review of Quantum Mechanics: 1. 2. 3. 4. The Schrodinger Equation. The Simple Harmonic Oscillator. Angular Momentum. The Hydrogen Atom. 2.2, 2.5, 3.1-3.3 2+3+4 Approximation Methods: 1. Time-Independent RayleighSchrodinger Perturbation Theory. 2. Time-Dependent Rayleigh-Schrodinger Perturbation Theory. 2.8, also see Ch. 8 & 9 in quantum Mechanic by Bransden and Joachain 5+6+7 Interaction with Electromagnetic Radiation 1. The Electromagnetic Fields. 2. Transition Rates. 3. The Dipole Approximation. 4. The Einstein Coefficients. 5. Selection Rules. 6. Line Intensities. 4.1, 4.2, 4.3, 4.4, 5, 4.6 8+9+10 Interaction with External Electric and Magnetic fields and Fine Structure 1. The Strak Effect. 2. The Zeeman Effect. 3. Fine Structure of Hydrogen Atoms. 4. The Lamb Shift 5.1, 5.2, 5.3, 5.4 11+12 Two-Electron Atoms 1. The Schrodinger Equation of TwoElectron Atoms. 2. Spin Wave Function and Pauli Exclusion Principle. 3. The Ground State of Two-Electron Atoms. 4. The Excited State of Two-Electron Atoms. 13+14 Many-Electron Atoms 1. The General Field Approximation. 2. The Periodic System of the Elements. 3. Correction to the central field 6.1, 6.2, 6.3, 6.4, 6.5, 6.6 7.1, 7.2, 7.5 approximation and L-S coupling and j-j coupling. 15+16 Atomic Collisions: basic concepts and potential 1. Type of collisions, channels, thresholds and cross-sections. 2. Potential scattering, general features. 3. The method of partial waves. 4. The Born approximation. 11.1, 11.2, 11.3, 11.4 Additional Notes Assignments Homeworks Exams The final exam is a comprehensive exam. Cheating is prohibited According to the regulations of the university, there is a punishment to any student tries to cheat in the exam. Any student misses more than 10% of the lectures without accepted excuse will fail the course. Cheating Attendance Workload Graded Exams Participation Laboratory Projects










