Chemistry, study of the composition, structure, properties, and
advertisement

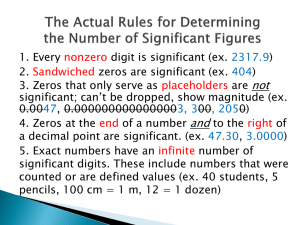
1 Chemistry Chemistry is the science that studies the composition, structure and properties, and interactions of matter I. Brief History Chemistry arose from attempts by people to transform metals into gold beginning about AD 100, an effort that became known as alchemy Modern chemistry was established in the late 18th century, as scientists began identifying and verifying through scientific experimentation the elemental processes and interactions that create the gases, liquids, and solids that compose our physical world. As the field of chemistry developed in the 19th and 20th centuries, chemists learned how to create new substances that have many important applications in our lives. Chemists, scientists who study chemistry, are more interested in the materials of which an object is made than in its size, shape, or motion. Chemists ask questions such as what happens when iron rusts, why iron rusts but tin does not, what happens when food is digested, why a solution of salt conducts electricity but a solution of sugar does not, and why some chemical changes proceed rapidly while others are slow. Chemists have learned to duplicate and produce large quantities of many useful substances that occur in nature, and they have created substances whose properties are unique. Much of chemistry can be described as taking substances apart and putting the parts together again in different ways. Using this approach, the chemical industry produces materials that are vital to the industrialized world. Resources such as coal, petroleum, ores, plants, the sea, and the air yield raw materials that are turned into metal alloys; detergents and dyes; paints, plastics, and polymers; medicines and artificial implants; perfumes and flavors; fertilizers, herbicides, and insecticides. Today, more synthetic detergent is used than soap; cotton and wool have been displaced from many uses by artificial fibers; and wood, metal, and glass are often replaced by plastics. reference: http://encarta.msn.com/encyclopedia_762504460/Chemistry.html#s57 II. Chemistry as Quantitative Science • The study of chemistry could involve both qualitative and quantitative aspect of materials around us. • The qualitative aspect involves describing materials such as color and the change they under goes. • The quantitative side concerned with measuring and calculating the characteristics of materials. This quantitative aspect has played, and continues to play, an important role in modern chemistry. • Much of chemistry can be described as taking substances apart and putting the parts together again in different ways. Using this approach, the chemical industry produces materials that are vital to the industrialized world. 2 Chemistry is an experimental science: base on the scientific method. The scientific method is the use of carefully controlled experiments to answer scientific questions (the combination is the combination of observation, experimentation, and the formulation of laws, hypothesis, and theories.) Observation (question): natural or experimental Tentative explanation: hypthesis Revise if experiments show hypothesis is inadequate Experimental designed to test hypothesis Theory that amplifies hypothesis and give prediction Modify theory if experiments show model is inadequate A hypothesis is a tentative explanation for an observation or a phenomenon.. If a hypothesis survives testing by experiments, it is often referred to as a theory. A theory is a model or way of lookingat nature that can be used to explain natural laws and make further predictions about nature phenomena. An experiment is an observation of natural phenomena carried out in a controlled manner so that the result can be duplicated and rational conclusions obtained. A law is a concise statement or mathematical equation about a basic relationship or regularity of nature. Experiments to test predication of theory Theory estiblished unless later experiments or observation show inadequacies of model Quick-Review Question III. Experimentation and Measure Measurement is the comparison of a physical quantity to be measured with a unit of measurement-that is a fixed standard of measurement. In 1960 the General Conference on Weights and Measures adopted the International System of units (SI units) which is particular choice of metric unit. This system has seven SI base units from which all others can be derived. One of the advantages of any metric system is that it is a decimal system. Quantities differing from the base unit by powers of ten are noted by the use of prefixes. 3 Table 1 The seven fundamental units of measure Physical quantity Name of unit Mass kilogram Length meter Temperature Kelvin Amount of substance mole Time second Electric current ampere Luminous intensity candela Table 2 Some SI Prefixes Multiple Prefix__ 15 10 peta 1012 tera 9 10 giga 106 mega 103 kilo 2 10 hecto 10 deca -1 10 deci 10-2 centi -3 10 milli 10-6 micro -9 10 nano 10-12 pico -15 10 femto Abbreviation kg m K mol s A cd_______ Symbol__ P T G M k h da d c m n p f_______ Mass Relation between mass and weight Weight is the force of gravity on an object. It is directly proportional to mass. Length The meter (m) is the standard unit of length in the SI system. 1 m = 100 cm = 1,000 mm = 1,000,000 m 4 Temperature To establish a temperature scale, we arbitrarily set certain fixed points and temperature increments called degree. Two commonly used fixed points are the temperature at which ice melt and the temperature at which water boils. The Celsius scale is the temperature scale in general scientific use. On this scale, the freezing point of water is 0C and the boiling point of water at normal barometer pressure is 100C. However, the SI base unit of temperature is the Kelvin (K), a unit on an absolute temperature scale. On this scale, the value zero is the lowest possible temperature can be obtained theoretically which is 273.15C, sometime called absolute zero. 0K = 273.15C. A comparison of the Kelvin, Celsius, and Fahrenheit temperature scale. Melting Point Boiling point of water E.g. The hottest place on record in North America is in Death Valley, California. A temperature of 134F was reached there in 1913. What is this temperature reading in degree Celsius? in Kelvin? Derived Units Area (m2), volume (m3), density (kg/m3), speed (m/s) etc are some of the derived quantities that you are familiar with. They are derived quantities rather than fundamental quantities because they can be expressed using one or more of the seven base units. Volume, the amount of space occupied by an object, is measured in SI units by the cubic meter (m3), defined as the amount of space occupied by a cubic 1 meter long on each edge. 5 Density Density is the intensity property that relates the mass of an object to its volume. Because most substances change in volume when heated or cooled, densities are temperaturedependent for example, the density of water at 3.98C is 1.000g /mL and at 100C is 0.9584 g/mL as the volume expand. Although most substances expand when heated and contract when cooled, water behaves differently. Water contract when cooled from 3.98C to 0C. E.g. 2) What is the density of glass if a sample weighing 62.0 g has a volume of 12.4 cm3? 6 IV. Working With Units: Dimensional Analysis Dimensional analysis is the method of calculation in which one carries along the units for quantities. Suppose we want to find the volume (V) of a cube, give l, the length of a side of the cube. Because V = l3, if l = 5.00cm, we find that V = (5cm)3 = 125cm3. There is no guesswork about the unit of volume here; it is cubic centimeter (cm3). Suppose, however, that we wish to express the volume in liter (L), a metric unit that equals 103 cubic centimeters. We can write this equality as 1L = 103 cm3. If we divide both sides of the equality by the right-hand quantity, we get Observe that units are treated in the same way as algebraic quantities. Note too that the righthand side now equals 1 and there are no units associated with it. Because it is always possible to multiply any quantity by 1 without changing that quantity, we can multiply our previous expression for volume by the factor 1 L/103 without changing the actual volume. We are changing only the way in which we express this volume: The ratio 1 L/ 103 cm3 is called a conversion factor because it is a factor equal to 1 that converts a quantity expressed in one unit to one expressed in another unit. Note that the numbers in this conversion factor are exact, because one liter equals exactly one thousand cubic centimeter. Such exact conversion factors do not affect the number of significant figures in an arithmetic result. E.g. Convert 8.45 kg to milligrams E.g. What is the density of a substance in g/mL, if a sample with a volume of 0.085 liters has a mass of 1700 mg? V. Uncertainties in Scientific Measurement All measurements are subject to error. Types of Errors: 1) Systematic error: arise because to some extent, measuring instruments have built-in, or inherent, errors. 7 2) Random errors: they arise from intrinsic limitation in the sensitivity of the instrument and inability of observer to read a scientific instrument and give results that may be either too high or too low. Accuracy and Precision In talking about the degree of uncertainty in a measurement, we use the words accuracy and precision. Accuracy refers to how close to the true value a given measurement is. Precision refers to how well a number of independent measurements agree with one another. There is no relationship between accuracy and precision, since an experiment can have small random errors and still give inaccurate results due to large systematic errors. E.g. E.g. Imagine that you weigh a tennis ball whose true mass is 54.44178 g. Assume that you take three independent measurements on each of three different types of balance to obtain data shown in the following table Measurement # Bathroom scale1 Platform balance2 Analytical balance 3 1 0.0 kg 54.4g 54.4419g 2 0.0 kg 54.7g 54.4417g 3 0.1 kg 54.1g 54.4417g average 0.03 kg 54.4g 54.4418g VI. Significant Figures To indicate the precision of a measured number (or result of a calculation with measured numbers), we often use the concept of significant figures. Significant figures are those digits in a measured number (or result of a calculation with measured numbers) that include all certain digits plus a final one having some uncertainty. When we measured the rod, we obtained the values 9.12 cm, 9.11 cm and 9.13 cm. We report the result as the average 9.12 cm. The first two digits (9.1) are certain, and the next digit (2) is estimated, so has some uncertainty. 8 The rules for determining significant figures (sig. fig.). Number of significant figures refers to the number of digits reported for the value of a measurement or calculated quantity, indicating the precision of the value. To count the number of sig. fig. in a given measured quantity, we observed the following rules: 1 Zeros in the middle of a numbers are significant figures. E.g. 4023 mL 2 Zeros at the beginning of a number are not significant; they act only to locate the decimal point. E.g. 0.00206L 3 Zeros at the end of a number and after decimal point are always significant. E. g. 2.200 g 4 Zeros at the end of number and before the decimal point may or may not be significant figures. In such cases we must deduce the number of sig. fig. from the statement of the problem. E.g. The statement “ 350,000 spectators lined the parade route” involves a number that probably has only two sig. fig. because it is obvious that no one actually counted the spectators. 5 A useful rule of thumb to use for determining whether or not zeros are significant figures is that zeros are not sig. fig. if the zeros disappear when scientific notation is used. Scientific Notation • Scientific Notation was developed in order to easily represent numbers that are either very large or very small. • For example, instead of writing 0.0000000056, we write 5.6 x 10-9. So, how does this work? • We can think of 5.6 x 10-9 as the product of two numbers: 5.6 (the digit term) and 10-9 (the exponential term). • A positive exponent shows that the decimal point is shifted that number of places to the right. A negative exponent shows that the decimal point is shifted that number of places to the left. • In scientific notation, the digit term indicates the number of significant figures in the number. The exponential term only places the decimal point. • 9 • E.g.1) 46600000 = 4.66 x 10 • E.g.2) 0.00053 = 5.3 x 10-4 • E.g.(3) Write in scientific notation: 0.000467 and 32000000 • E.g.(4) Express 5.43 x 10-3 as a number. Significant Figures in Numerical Calculations 1 Multiplication or Division The result of multiplicand or division, may contain only as many sig. fig. as the least precisely known quantity in the calculation. If use scientific notion, the digit terms are multiplied in the normal way and the exponents are added. The end result is changed so that there is only one nonzero digit to the left of the decimal. E.g. (3.4 x 106)(4.2 x 103) The digit terms are divided in the normal way and the exponents are subtracted. The quotient is changed (if necessary) so that there is only one nonzero digit to the left of the decimal. E.g. (6.4 x 106)/(8.9 x 102) • • • 2 Addition and Subtraction: The result of addition or subtraction must be expressed with the same digits beyond the decimal point as the quantity carries the smallest number of such digits. 10 • • • • • • • • • • • • When use scientific notion, all numbers are converted to the same power of 10, and the digit terms are added or subtracted. E.g. (4.215 x 10-2) + (3.2 x 10-4) 3 Exact numbers can be considered to have an unlimited number of sig. fig. There are two situation when a quantity appearing in a calculation may be exact. By definition such as 1 in = 2.54 cm Counting such as six face on a cube or tow hydrogen atoms in a water molecule. Powers of Exponentials: The digit term is raised to the indicated power and the exponent is multiplied by the number that indicates the power. E.g. (2.4 x 104)3 Roots of Exponentials: Change the exponent if necessary so that the number is divisible by the root. Remember that taking the square root is the same as raising the number to the one-half power. E.g. VII. Rounding Numbers