Congenital Melanocytic nevus
advertisement

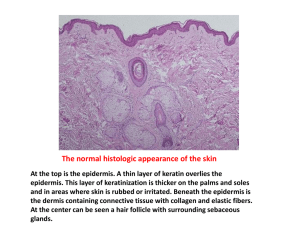
Congenital Melanocytic Nevus Defined as benign nevomelanocytic proliferations present at birth. Rarely, melanocytic nevi with features indistinguishable clinically and histologically from typical congenital melanocytic nevi appear in children between 1 month and 2 years of age. This subset is termed nevus tardive Classification Small Intermediate Large <1.5cm 1.5-19cm >20cm >2%BSA or 1%SA of Head Clinical appearance Generally, congenital melanocytic nevi are round to oval in shape and have a regular, smooth, and well-demarcated border. After the first 6 months, the lesions grow proportionally to the particular area of the body involved Clinical appearance may change with age. In neonates, the moles may be light in color and relatively hairless, with a flat or raised surface. As the child grows, the nevi may become progressively darker, with a uniform brown to dark brown or black color and may acquire long, coarse, darkly pigmented hairs. May exhibit a papular, rugose, pebbly, verrucous, or even cerebriform surfaces Satellite smaller melanocytic nevi may also be present, “Kissing nevus,” or divided nevus of the eyelid, is an interesting clinical variant of congenital melanocytic nevi. It occurs on adjacent parts of the upper and lower eyelids and appears contiguous, as a single lesion, when the eyelids are closed. This finding implies that congenital melanocytic nevi develop between the 9th and 20th week of gestation, when the eyelids are fused Histology 1. nevomelanocytes splaying or extending between the collagen bundles of the reticular dermis as single cells, “Indian” files, or cords of cells 2. nevus cells in lower 2/3rd dermis 3. extension of nevomelanocytes around and within hair follicles, sebaceous glands, eccrine apparatus, vessel walls, and nerves 4. perivascular and perifollicular distribution of nevomelanocytes simulating an inflammatory reaction such as figurate erythema 5. arrector pili that may be enlarged, distorted, and infiltrated by nevomelanocytes. Atypical features of congenital nevi include 1. dysplasia of intraepidermal nevomelanocytes 2. pagetoid spread of nevomelanocytes - pagetoid intraepidermal growth pattern in benign melanocytic nevi is confined to the area of nested intraepidermal proliferation, in contrast to melanoma in which the pagetoid growth is observed as lateral spread beyond the nested proliferation 3. proliferative dermal nodules. Distinctive “Indian file” array of nevomelanocytes. During the first 6 months of life, some nevi can appear to ‘‘grow’’ significantly as tardive pigment becomes more visible. Some satellite nevi may become visible for the first time over the first 2 to 3 years (tardive CM) Associations scoliosis, spina bifida, clubfoot, cranial bone hypertrophy Neurocutaneous melanosis First described by Rokitansky presence of giant (2/3rd) or multiple(1/3rd) melanocytic nevi associated with benign or malignant melanotic tumors of the central nervous system. posterior axial location, especially when associated with "satellite" melanocytic nevi at greatest risk Called leptomeningeal melanocytosis with CMS melanoma Leptomeningeal melanoma present in upto 62% of the cases, but even in the absence of melanoma, symptomatic neurocutaneous melanosis has an extremely poor prognosis. Patients with large or multiple congenital melanocytic nevi in axial locations (head, neck, or posterior midline) or multiple satellite lesions seem to have an increased risk of leptomeningeal melanocytosis. Most case are sporadic, and no gender or racial predilection is evident. Melanocytes of both skin and leptomeninges are thought to be derived from multiple potential precursor cells of the neural crest, NCM is postulated to represent a congenital error in embryonic neuroectoderm morphogenesis. CSF shows increased protein, decreased sugar, and a normal cell count.. May show melanin-filled cells Criteria for the diagnosis of neurocutaneous melanosis: (1) The presence of large or multiple congenital melanocytic nevi (one of which is at least 20 cm in diameter) with benign (melanosis) or malignant (melanoma) central nervous system (CNS) tumors. (2) The absence of malignant melanoma in any organ (including skin) other than the CNS. Histology accumulation of melanotic cells in the arachnoid and pia mater Parenchymal melanin deposits probably represent melanocytes in the perivascular spaces. Clinical Hydrocephalus – usually secondary to meningeal thickening, Seizures Mass lesions Cranial nerve palsies Mental retardation Spinal cord compression Investigations MRI – T1 shortening characteristic CSF cytology Prognosis death occurred in more than half the patients within 3 years of the onset of neurological symptoms, most <10 years old Outcome 2 main concerns 1. Risk of melanoma 2. Stigma of visible lesion and how it will affect psychosocial development Risk of melanoma In general, risk is related to nevomelanocytic load In several case series, no melanoma noted in small and intermediate lesions Approximately 50% of the malignancies that develop in large CMN do so in the first 3 years of life, and 70% by puberty (<10yr old) Giant melanoma – 2 prospective studies i. Ruiz-Maldonado (1992): Three of 80 patients (3.8%) developed cutaneous melanoma during an average follow-up period of 4.7 years ii. Marghoob (1996): Ninety-two patients were followed up prospectively for an average of 5.4 years. Melanoma developed in 3 patients (3.3%) in extracutaneous sites. iii. Watt (PRS 2004) – review of 8 studies. Observed incidence of melanoma was 2600 times higher than would be expected. majority of melanomas that arise in association with large or giant congenital melanocytic nevi originate in the dermis. They are usually composed of epithelioid cells, spindle cells, or small round cells. Melanomas arising in small congenital melanocytic nevi usually originate in the epidermis To date, no case of melanoma has been reported arising in a satellite nevus Treatment Rationale for early treatment 1. greatest risk of melanoma during early years 2. elasticity and healing capacity of the skin in the early years 3. more tolerant to surgery /tissue expansion 4. psychological benefit Treatment options 1. observation 2. dermabrasion 3. curettage 4. chemical peel 5. Q-switched ruby laser 6. staged excision and reconstruction Dermabrasion and curettage remove the more concentrated population of nevus cells near the lesion’s surface. Theory is that the nevus cell migrate deeper with age can be effective in reducing the overall nevus ‘‘cell load’’ but cannot fully remove the nevus, because of the well-known depth of nevus cells in CMN may result in significant lightening of the color of the lesion, it is quite common to see later ‘‘bleed-through’’ of the deeper nevus, with gradual darkening and reappearance of the lesion. lightening of pigmented giant congenital nevi using these techniques may make it more difficult to monitor the resultant lesion for signs of malignant transformation, because alteration in pigmentation of the lesion can no longer be followed reliably. no longitudinal studies documenting decreased rates of malignant transformation after laser or dermabrasion treatment. Reconstruction there is no evidence in the existing literature that the excision of large congenital melanocytic nevi decreases the prevalence of melanoma. Serial excision undertaken if lesion can be removed in 3-4 excisions. Otherwise tissue expand. SSG SSG or full thickness grafts(preferred) o FTSG donor sites – postauricular, supraclavicular, buttock, groin, medial arm, abdomen o May be expanded to increase donor area o Use of Integra at recipient site may improve scar Tissue expansion (Bruce Baeur) Advantage - large flaps of color-, thickness-, and texture-matched skin while simultaneously minimizing donor-site defects. optimized to ensure that the landmarks of the forehead aesthetic unit will be disturbed as little as possible. Particular emphasis is placed on brow symmetry, temporal hairline position and hair direction, and scar orientation. Scalp Treatment starts as early as 6 months, with some cranial molding expected by the time the expanders are removed No instance of long-term cranial deformity has been noted (remodeling usually occurs over 3 to 4 months). Rectangular expanders with remote ports, placed subgaleal (250-500ml size) Usually 2 expanders used Expanded weekly, 2nd stage in 10 weeks Face Unilateral forehead – hemiforehead expansion Central forehead – bilateral forehead expansion o Expanders placed subfrontalis o Because many of these nevi involve the adjacent scalp, the combined ‘‘attack’’ on both of these regions often facilitates the excision and aesthetic outcomes Cheek – postauricular and neck flaps Eyelids – best with FTSG, donor site may be preexpanded to increase donor area and reduce seams. Brow can be reconstructed with island flaps from temporalis (below), or hair bearing FTSG or with micrograft hair transplants Torso Early expansion successful for lower or central abdomen, back and buttocks Expansion must be avoided in or around the area of the breast bud in females, and lesions of the breast should be left until after breast development, regardless of the psychological implications of delaying the treatment till that age. Bathing trunk nevus – reconstruct only areas in which reconstruction can reasonably be expected to provide a better aesthetic and functional outcome than the original lesion, even if this means leaving residual nevus in the genital and perineal regions Extremities Serial excision is the preferred treatment modality for lesions at or just proximal to the knee or elbow. Similar lesions distal to the knee or elbow are difficult to treat using serial excision alone because of reduced skin compliance and reduced diameter of the distal extremity. Expanded skin grafts have been used but long-term follow-up of these patients typically demonstrated contour deformities and unacceptable aesthetic outcomes. There are also concerns about both the durability of these grafts and their ability to keep up with normal extremity growth. Expanded full-thickness skin grafts have been used effectively for the dorsum of the hand, with excellent aesthetic outcomes geometry of the extremity, as well as the limited flexibility of the skin (particularly in the lower extremity) makes regional expansion of limited use. Skin grafts generally used below the knee In selected cases o large expanded transposition flaps from the scapular region to cover the upper arm and shoulder and expanded or nonexpanded pedicle flaps from the flank and abdomen for circumferential nevi from the elbow to the wrist. Summary Early surgical intervention with multiple staged excisions with tissue expansion and or grafting is the best approach as well as close clinical follow-up for the remaining areas that are too difficult to be removed or in areas that would produce too much scaring.