measurements, units & calculations
advertisement

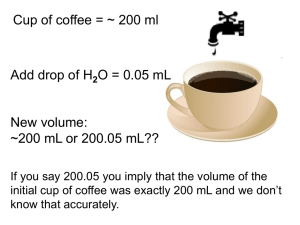
MEASUREMENTS, UNITS & CALCULATIONS Chemistry is a quantitative science: The outcome of an experiment depends on amount of substances taken, temperature, pressure, & duration of the reaction. QUALITATIVE vs. QUANTITATIVE Properties Qualitative: color, smell, ability to undergo chemical transformation of a certain kind. Quantitative: properties that are expressed using number & unit: mass, volume, temperature, time, distance, etc. Often properties previously considered as qualitative only, are then characterized as quantitative, e.g. color is related to spectrum that is expressed in numbers & units. Properties to be measured most often: MASS, m SIZE as volume, V TEMPERATURE, toC or T, K TIME, t To perform a measurement, one need an INSTRUMENT & a STANDARD e.g. for mass: a balance & a standard load of 1 kg (Pt cube kept in Sevres, in France, to which all other standards are compared) for distance: a ruler, caliper, etc. & a standard meter; for time: a clock & a duration of some physical process for which we know that it is always the same; for temperature: a thermometer & some standard process, such as ice melting (taken as 0oC) & water boiling (taken as 100oC). The result of a measurement is expressed by a NUMBER & a UNIT: mass of body: 188 lb or 84 kg Thus, we need to know how to deal with numbers & units, according to certain rules providing reliable representation of the results of measurements & calculations. SIGNIFICANT DIGITS IN THE RESULTS OF DIRECT MEASUREMENTS Uncertainty in Measurements - All scientific measurements are uncertain, i.e. subject to error. - Due to errors, two successive measurements of the same quantity are different. - These errors are reflected in the number of figures (digits) reported for the measurement. SIGNIFICANT DIGITS IN THE RESULTS OF DIRECT MEASUREMENTS All digits in which we are certain, & the next one, which is known tentatively (estimated) are significant Example: Measuring volume with a graded beaker. Minor grade is 5 mL. The volume may be estimated as 47 mL, with 2 sd In 2 digits we are certain, the 3rd one is an estimation Measuring volume with a graded cylinder _ Minor grade is 1 mL. Estimated volume is 27.0 mL 3 s.d. Here estimated volume is 36.5 mL 30 25 20 15 10 5 SIGNIFICANT DIGITS in DATA GIVEN: Non-zero digits are always significant. 22 has 2 significant digits, 0.2234 - 4 s. d. 22.3 has 3 s. d. 0.00234 – 3 s. d. Numbers with zeroes: Zeroes before all other digits are not significant; 0.046 has 2 s. d. 45.26 ft - 4 s.d. Zeroes between other, non-zero, digits are always significant; 4009 kg 4 s. d. 35.054 5 s. d. Zeroes after other digits but behind a decimal point are significant; 7.90 has 3 s. d. 8.20 103 has 3 s. d. 8.200 103 has 4 s. d. 8.2 103 has 2 s. d. It is impossible to tell if zeroes are significant when they stand at the end of a number with no decimal point. Apply your common sense. 8200: it is not clear if the zeroes are significant or not. The number of significant digits in 8200 is at least two, but could be three or four. USA population is estimated as 295 734 000. Obviously, it cannot be known exactly (due to continuous migrations, birth & death). Most likely, there are 6 s.d. Integer numbers & coefficients given by definition have infinite number of s. d. e.g. 1 ft = 12 inches 1 km = 1000 m are considered definition, hence infinite number of s.d. SIGNIFICANT DIGITS IN CALCULATIONS Basic principle: information cannot be created by calculations. If some quantity is known not precisely, this lack of precision will propagate through calculations. 1. For multiplication & division, the number of SD in the result is the same as that in the number with the smallest number of SD 27.9/3.0 = 9.3 (not 9.30 !) 160 2.16 = [345.6] = 350 Sometimes, to indicate that the last zero is significant, the number is terminated by a dot: 160. 2.16 = [345.6] = 346 2. For addition & subtraction, the number of digits after decimal point in the result is the number of decimal places (i.e. after decimal point) in the term with the smallest number of decimal places: 2.345 + 33.11 + 210.2 = 245.7 2100 + 0.45 = 2100 (sic!) 3 2.100 10 + 1.56 = [2101.56] = 2102 ROUND OFF If the digit to be removed is <5 (midway pnt), the preceding digit stays the same. If it is >5 (even in the next digit), the preceding digit is increased by 1. If it is = 5, the agreement is to go to the closest even number (up or down): to 3 SD: 9.374 = 9.37; 4.345 = 4.34 4.3451 = 4.35; 4.355 = 4.36 in math, always, 2000 + 1 = 2001 in science, sometimes it is still 2000! SCIENTIFIC NOTATION is based on powers of the base number 10, i.e. 100 =1 (true for any number, not only 10: 20 =1, 450 =1, etc.) 101 = 10 10-1 = 0.1 102 = 100 10-2 = 0.01 103 = 1000 10-3 = 0.001 …………… …………….. Sci. notation is used to present very large (approx. >1000) & very small (approx <0.001) numbers. The number 123,000,000,000 in scientific notation is written as: 1.231011 The first number 1.23 is the coefficient. It must be >1 but < 10. The second number, 10, is the base. In the number 1.23 1011 the number 11 is referred to as the exponent or power of ten. Other notations: The number 123,000,000,000 can also be written as: 1.23E+11 0.00000683 = 6.83 10-6 or as 6.83E-6 1.23 10^11 6.83 10^(-6) Rules for Multiplication/Division in Scientific Notation: - Multiply the coefficients - Add the exponents (subtract if negative) (3 104) (2 105) = 6 109 Examples: If the coefficient is > 10, it must be converted: (6 104) (2 105) = 12 109 = 1.2 101 109 = 1.2 1010 The number of particles in a mole is NAv = 6.0231023; in 2 moles: 26.0231023 = 12.051023= 1.2051024. Mass of one hydrogen atom in gram is: 1/NAv = 1/6.0231023 = 0.1660 10-23 = 1.660 10-1 10-23 = 1.660 10-24 g (6 x 106) (2x 103) (2x 103) ________________________________ ______4 x 10 4 6x2x2 = ________________ 106+3+3-4 = 6 x 108 4 Rules for Addition/Subtraction in Scientific Notation: Before adding/subtracting, the numbers must be presented as the same power of 10. Then, the coefficients are added/subtracted, & the power of 10 does not change. Example: 1.73104 + 9.83103 =17.3103 +9.83103 = (17.3+9.83)103 = 27.13103= 2.7 104 Note: if a very small number is added to a very large one, the large one does not change: 1.73 104 + 9.83 10-3 = 1.73 104 Power to power in scientific notations: (ax) y = axy (102)3 = 106 Example: what is the volume (in cm3) of a sodium atom with its atomic radius of 1.9 10-8cm? V = (4/3)r3 = (4/3) 3.14 1.9 10-8)3 = 1.33 3.14 (1.9)310-8)3 = = 28.64 10-24 = 2.9 10-23 cm3 UNI T S In science, metric International System of units is used (SI). Its basic units are those of: Length (meter) Mass (kilogram) Time (second) Temperature (K) SI is a metric system. Other units of length, mass & time are n produced using decimal factors, 10 , integer n, & are named using Greek numeral prefixes. deci for 10-1: 1dm = 10-1m centi 10-2: 1cm = 10-2m milli 10-3: 1mg = 10-3g micro 10-6: 1s = 10-6s nano 10-9: 1nm = 10-9m kilo 103: 1km = 103m mega 106: 1MW=106 W (Watt) pico 10-12: 1ps = 10-12s femto 10-15: 1fs = 10-15s Length, mass, temperature & time are considered as basic properties, & their units are considered as basic, or simple. Others are DERIVATIVE or complex units: Volume of a cube is l3, hence V[m3]. In chemistry, smaller unit is used: 1 L = 1 dm3 = 1000 mL, 1 mL = 1cm3 Example: what is the volume (in cm3) of a sodium atom with its atomic radius of 1.9 10-8cm? V = (4/3)r3 = (4/3) 3.14 1.9 10-8)3 = 1.33 3.14 (1.9)310-8)3 = = 28.64 10-24 = 2.9 10-23 cm3 The choice of units is a matter of convenience: Density, D = m/V, hence, D[kg/L] = g/mL -used for condensed matter or D[g/L], for gases transp Densities of some materials: Liquids & Solids, D[g/mL] Dry Wood Ethanol Vegetable oil Water Sugar Magnesium Salt Lead Gases D[g/L] (standard temp & pressure) Hydrogen Helium Methane Neon CO2 Air Oxygen HCl 0.512 0.789 0.91 1.00 1.59 1.74 2.16 11.34 Speed, l/t [m/s] or [km/hr] Three temperature scales: Celsius, Kelvin & Fahrenheit. temperature kelvins degrees Celsius degrees Fahrenheit symbol K °C °F boiling point of water 373 100. 212. melting point of ice 273 0. 32. absolute zero 0 -273 -459 Temperature reflects the internal motion of particles. T = 0 K is temperature at which all motion stops. This is the absolute zero of T, K (it cannot be negative!) The value of |1K| = |1°C|, but these two scales are shifted against each other by 273: T, K = t°C + 273; or t°C = T K – 273 The interval between water freezing & boiling is 100°C, but 212-32 = 180°F, Hence the value of |1°C| = |1.8°F|, or 9/5 °F & conversion rule is: t°C = (5/9)(t°F - 32) [roughly: ½ (t°F - 32)] & t°F = (9/5) t°C + 32 [roughly: 2t°F + 32)] Example: At what temperature its values in °F & in °C coincide? t°F = (9/5) t°C + 32 t = (9/5)t + 32 t = -40, i.e. t = -40°F = -40°C UNIT CONVERSION Example: Speed 110. km/hr. What is this speed in mi/hr & m/s? From the table: 1 mi = 1.61 km Also, we know: 1 hr = 60 min = 6060 =3600 s. It is always possible to 1 (UNIT FACTOR METHOD). from 1 mi = 1.61km, it follows: 1.61km /1mi = 1 Speed(mi/hr) = (110 km/hr) (1mi/1.61km) = 68.3mi/hr Speed(m/s)=(110 km/hr)(103m/km)(1hr/3600s) = 30.6m/s At unit conversion, the number of s.d. does not change. “rails” may help in formatting those calculations: 110 km 103m 1 hr Speed(m/s) = = 30.6 m/s hr 1 km 3600 s ACCURACY & PRECISION ACCURACY refers to the closeness of a measurement to the true or accepted value of the quantity measured. PRECISION refers to the agreement between the numerical values of a set of measurements of the same quantity made in the same way. PERCENT ERROR = |Value accepted - Value obtained| 100% Value accepted Not accurate not precise Accurate but not precise Precise but not accurate Accurate & precise Example: Aluminum density, from a handbook, is D = 2.71 g/mL In an experiment, you got 2.63 g/mL. What is the percent error? (2.71 - 2.63) / 2.71 100% = 3.0% This is the measure of your accuracy. The result is accurate within 3%. The results my be accurate but not precise, & vice versa. Example: In 4 trials, the aluminum density was found as 2.63, 2.82, 2.79 & 2.51. What is the precision of these measurements? 1. Calculate the mean, Dm: Dm = (2.63+2.82+2.79+2.51)/4 2.68 g/mL 2. Deviations from the Dm are: D1 = |2.63-2.68| = 0.05 [absolute values are taken] D2 = |2.82-2.68| = 0.14 D3 = |2.79-2.68| = 0.11 D4 = |2.51-2.68| = 0.17 3. Mean deviation: Dm = (0.05+0.14+0.11+0.17)/4 0.12 The result may be presented as: D = Dm ± Dm = (2.68 ± 0.12) g/mL Dm is the measure of precision. It may be presented as percent deviation: (Dm/Dm).100% = (0.12/2.68) 100% = 4.5% What is the accuracy of the above set of data? Handbook data: D(Al) = 2.71 g/mL The mean value of 4 measurements was Dm = 2.68 g/mL Percent error = |2.68-2.71|/2.71100% = 1.1%