Solutions
advertisement

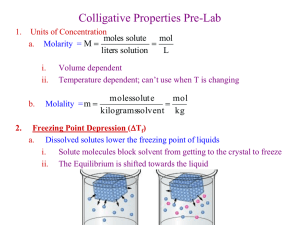
Solutions Chang 7th Edition Chapter 12 General Chemistry II W hy does a raw egg swell or shrink when placed in different solutions? Chapter Objectives Define solution, solvent, solute and colligative properties and use them in calculations to solve for molality, mole fraction, weight percent and ppm Explain the differences between saturated, unsaturated and supersaturated solutions, miscible and immiscible Use lattice energy and enthalpy of hydration to explain enthalpy of solution Use Henry's law and Le Chatelier's principle to explain solubility of gases Solve problems using mole fraction, Raoult's law, and calculations predicting changes in colligative properties, and solving for variables related to colligative properties Define and calculate Osmotic pressure Driving forces for solution formation Some Definitions A solution is a HOMOGENEOUS mixture of 2 or more substances in a single phase. One constituent is usually regarded as the SOLVENT and the others as SOLUTES. 4 major topics Ways to describe solution concentrations (amount of solute per unit of solution) How and why solutions form Colligative properties (those properties that depend on the number of solute particles per solvent molecule - but not on the identity of the solute) Colloids Concentration Units An IDEAL SOLUTION is one where the properties depend only on the concentration of solute. Need conc. units to tell us the number of solute particles per solvent particle. The unit “molarity” does not do this! Concentration Units MOLE FRACTION, X For a mixture of A, B, and C Concentration Units MOLE FRACTION, X For a mixture of A, B, and C MOLALITY, m Concentration Units MOLE FRACTION, X For a mixture of A, B, and C MOLALITY, m W EIGHT % = grams solute per 100 g solution Calculating Concentrations 687320265 page 1 of 8 Dr. Myton, CH116 Dissolve 62.1 g (1.00 mol) of ethylene glycol in 250. g of H2O. Calculate mol fraction, molality, and weight % of glycol. Calculating Concentrations 250. g H2O = 13.9 mol Calculating Concentrations 250. g H2O = 13.9 mol X glycol = 0.0672 Calculating Concentrations Calculate molality Calculating Concentrations Calculate molality Calculate weight % Problem Solving 12.33 A solution of ethyl alcohol, CH3CH2OH, in water has a concentration of 1.25 m. Calculate the weight percent of ethyl alcohol 12.34 Calculate the molarity of an aqueous solution of NaCl with a concentration of 0.363 m and a density of 1.0185 g/mL Problem Solving 12.37 A solution of ammonia in water is at a concentration of 5.00% w/w. Its density is 0.9787 g/mL, calculate the molarity and molality of the solution. Problem Solving Assume you add 1.2 kg of ethylene glycol HOCH2CH2OH as an antifreeze to 4.0 kg of water in the radiator of your car. What are the mole fraction, molalaity, molarity, weight percent and ppm concentrations of the solution? Problem Solving You dissolve 560 g of NaHSO4 in a swimming pool that contains 4.5e5 L of water at 25 ºC. What is the sodium ion concentration in ppm? Problem Solving If you dissolve 10.0 g of sugar C12H2O11 (about one heaping teaspoon) in a cup of water (250. G) what are the mole fraction, molarity, weight percent and ppm concentrations of the solution Problem Solving Sea water has a sodium ion concentration of 1.08e4 ppm. If the sodium is present in the form of dissolved sodium chloride, how many grams of NaCl are in each liter of sea water? Assume the density of sea water is 1.05 g/mL. Definitions Solutions can be classified as unsaturated or saturated. Definitions Solutions can be classified as unsaturated or saturated. A saturated solution contains the maximum quantity of solute that dissolves at that temperature. Definitions Solutions can be classified as unsaturated or saturated. 687320265 page 2 of 8 Dr. Myton, CH116 A saturated solution contains the maximum quantity of solute that dissovles at that temperature. SUPERSATURATED SOLUTIONS contain more than is possible and are unstable. Solutions and IM forces Bond breaking is endothermic Bond formation is exothermic Types of bonds: solute-solute, solvent-solvent, and solute-solvent An IDEAL SOLUTION the enthalpy of solution is zero Energetics of the Solution Process If the enthalpy of formation of the solution is more negative that that of the solvent and solute, the enthalpy of solution is negative. The solution process is exothermic! Supersaturated Sodium Acetate One application of a supersaturated solution is the sodium acetate “heat pack.” Sodium acetate has an ENDOthermic heat of solution. Supersaturated Sodium Acetate Sodium acetate has an ENDOthermic heat of solution. NaCH3CO2 (s) + heat ----> Na+(aq) + CH3CO2-(aq) Therefore, formation of solid sodium acetate from its ions is EXOTHERMIC. Na+(aq) + CH3CO2-(aq) ---> NaCH3CO2 (s) + heat Enthalpy of Solution Determine the heat of solution for ammonium nitrate (used in cold packs) Calculate the enthalpy of solution for sodium hydroxide (lye) Enthalpy Dissolving Gases & Henry’s Law Gas solubility Sg = kH • Pgas kH for O2 = 1.66 x 10-6 M/mmHg W hen Pgas drops, solubility drops. Henry’s Law Constants At 25 ºC Gas kH (M/ mm Hg) N2 8.42e-7 O2 1.66e-6 CO2 4.48e-5 Problem Solving What is the concentration of oxygen in a fresh water stream in equilibrium with the atmosphere at 25 ºC and 1.00 atm? Express your answer in ppm. What is the concentration of carbon dioxide in equilibrium with an atmospheric partial pressure of 0.33 atm at 25 ºC? Problem Solving At 740 torr and 20C, nitrogen has a solubility in water of 0.018 g/L. At 620 torr and 20C, its solubility is 0.015 g/L. Does nitrogen obey Henry’s law? Temperature dependence of solubility Colligative Properties On adding a solute to a solvent, the props. of the solvent are modified. Vapor pressure decreases Melting point decreases Boiling point increases Osmosis is possible (osmotic pressure) These changes are called COLLIGATIVE PROPERTIES. 687320265 page 3 of 8 Dr. Myton, CH116 They depend only on the NUMBER of solute particles relative to solvent particles, not on the KIND of solute particles. Releasing pressure changes the solubility of a gas in solution Lake Nyos, Cameroon Courtesy of George Kling, page 656-657 Understanding Colligative Properties To understand colligative properties, study the LIQUID-VAPOR EQUILIBRIUM for a solution. Understanding Colligative Properties To understand colligative properties, study the LIQUID-VAPOR EQUILIBRIUM for a solution. Adding a solute lowers vapor pressure Understanding Colligative Properties VP of H2O over a solution depends on the number of H2O molecules per solute molecule. Psolvent proportional to Xsolvent OR Psolvent = Xsolvent • Posolvent VP of solvent over solution = (Mol frac solvent)•(VP pure solvent) RAOULT’S LAW Raoult’s Law An ideal solution is one that obeys Raoult’s law. PA = XA • PoA Because mole fraction of solvent, XA, is always less than 1, then PA is always less than PoA. The vapor pressure of solvent over a solution is always LOW ERED! Raoult’s Law Assume the solution containing 62.1 g of glycol in 250. g of water is ideal. W hat is the vapor pressure of water over the solution at 30 oC? (The VP of pure H2O is 31.8 mm Hg; see App. G) Solution Xglycol = 0.0672 and so Xwater = ? Because Xglycol + Xwater = 1 Xwater = 1.000 - 0.0672 = 0.9328 Pwater = Xwater • Powater = (0.9382)(31.8 mm Hg) Pwater = 29.7 mm Hg Problem Solving Ethylene glycol, HOCH2CH2OH is a common ingredient in automobile antifreeze. If 651 g of ethylene glycol is dissolved in 1.50 kg of water (represents a typical 30.2% solution), what is the vapor pressure of water over the solution at 90 ºC? The vapor pressure of water at 90 ºC is 535.8 mm Hg, assume ideal behavior Problem Solving Assume you dissolve 10.0 g of sugar (C12H22O12 in 225 mL of water and warm the water to 60 ºC. What is the vapor pressure of the water over this solution at equilibrium? Psolvent = -Xsolute Pºsolvent Problem solving 687320265 page 4 of 8 Dr. Myton, CH116 12.43 The vapor pressure of water at 20C is 17.5 torr. A 20% by weight solution of ethylene glycol HOCH2CH2OH in water is prepared. Assuming that the solute is nonvolatile, do a calculation to estimate the vapor pressure of the solution Problem Solving Example 12.5 CCl4 has a vapor pressure of 100 torr at 23 degrees Celsius. Assume candle wax has a molecular weight of 331 and calculate the vapor pressure of carbon tetrachloride in a solution made of 10.0 g of the nonvolatile wax in 40.0 g of carbon tetrachloride Problem Solving 12.45 Benzene and toluene help get good engine performance from lead-free gasoline. At 40C the vapor pressure of benzene is 180 torr and that of toluene is 60 torr. To prepare a solution of these that will have a total vapor pressure of 96 torr at 40C requires what mole percent concentration of each? Raoult’s Law For a 2-component s ystem where A is the solvent and B is the solute DP PA = VP lowering = XBPoA VP lowering is proportional to mol frac solute! For very dilute solutions, DP PA = K•molalityB where K is a proportionality constant. This helps explain changes in melting and boiling points. Boiling Point Elevation Tbp = Kbp msolute The boiling point of a solution is higher than that of the pure solvent. BP/FP Elevation/Depression Constants Solvent BP Kbp ºC ºC/m FP water 100 +0.5121 0.0 -1.86 Benzene 80.10 +2.53 5.50 -5.12 Kfp ºC ºC/m Camphor 207.4 +5.611 179.75 -39.7 Change in Boiling Point Dissolve 62.1 g of glycol (1.00 mol) in 250. g of water. W hat is the BP of the solution? KBP = +0.512 oC/molal for water (see Table 14.3). Solution 1. Calculate solution molality = 4.00 m 2. DttBP = KBP • m DttBP = +0.512 oC/molal (4.00 molal) DttBP = +2.05 oC BP = 102.05 oC Problem Solving Eugenol, the active ingredient in cloves, has the formula C10H12O2. What is the boiling point of a solution when 0.144 g of this compound is dissolved in 10.0 g of benzene What quantity of ethylene glycol, HOCH 2CH2OH, must be added to 125 g of water to raise the boiling point by 1.0 ºC? Using BP for MW A solution prepared from 1.25 g of oil of wintergreen (methyl salicylate) in 99.0 g of benzene has a boiling point of 80.31 ºC. Determine the molar mass of methyl salicylate. Change in Freezing Point The freezing point of a solution is LOW ER than that of the pure solvent. FP depression = DttFP = KFP•m Freezing Point Depression Consider equilibrium at melting point Liquid solvent <------> Solid solvent 687320265 page 5 of 8 Dr. Myton, CH116 • Rate at which molecules go from S to L depends only on the nature of the solid. • BUT — rate for L ---> S depends on how much is dissolved. This rate is SLOW ED for the same reason VP is lowered. • Therefore, to bring S ---> L and L ---> S rates into equilibrium for a solution, T must be lowered. Thus, FP for solution < FP for solvent FP depression = DttFP = KFP•m Freezing Point Depression Calculate the FP of a 4.00 molal glycol/water solution. KFP = -1.86 oC/molal (Table 14.4) Solution DttFP = KFP • m = (-1.86 oC/molal)(4.00 m) DttFP = -7.44 oC Freezing Point Depression How much NaCl must be dissolved in 4.00 kg of water to lower FP to -10.00 oC?. Solution Calc. required molality DttFP = KFP • m -10.00 oC = (-1.86 oC/molal) • Conc Conc = 5.38 molal Freezing Point Depression How much NaCl must be dissolved in 4.00 kg of water to lower FP to -10.00 oC?. Solution Conc req’d = 5.38 molal This means we need 5.38 mol of dissolved particles per kg of solvent. Recognize that m represents the total conc. of all dissolved particles. Recall that 1 mol NaCl(aq) --> 1 mol Na+(aq) + 1 mol Cl(aq) Freezing Point Depression How much NaCl must be dissolved in 4.00 kg of water to lower FP to -10.00 oC?. Solution Conc req’d = 5.38 molal W e need 5.38 mol of dissolved particles per kg of solvent. NaCl(aq) --> Na+(aq) + Cl-(aq) To get 5.38 mol/kg of particles we need 5.38 mol / 2 = 2.69 mol NaCl / kg 2.69 mol NaCl / kg ---> 157 g NaCl / kg (157 g NaCl / kg)•(4.00 kg) = 629 g NaCl Problem Solving How many grams of ethylene glycol, HOCH2CH2OH, must be added to 5.50 kg of water to lower the freezing point of the water from 0.0 ºC to -10.0 ºC? Problem Solving Some people have summer homes on a lake or in the woods. In Northern climates these homes may be closed for winter and antifreeze added to the toilet tank to prevent damage from water freezing in the trap. Will adding 525 g of ethylene glycol, HOCH2CH2OH, prevent freezing at -25 ºC? Problem Solving Glycerol C3H8O3 (molecular mass 92) is essentially a nonvolatile liquid that is very soluble in water. A solution is made by dissolving 46.0 g of glycerol in 250 g of water. By calculations, estimate the following the boiling point of the solution at 1 atm its freezing point its vapor pressure at 25C (at this temperature the vapor pressure of water is 23.8 torr) Boiling Point Elevation and Freezing Point Depression 687320265 page 6 of 8 Dr. Myton, CH116 Dtt = K • m • i A generally useful equation i = van’t Hoff factor = number of particles produced per formula unit. Compound Theoretical Value of i glycol 1 NaCl 2 CaCl2 3 Problem Solving A 0.00200 m aqueous solution of an ionic compound Co(NH3)5(NO2)Cl freezes at -0.00732 ºC. How many moles of ions does 1 mole of the salt give upon being dissolved? Calculate the freezing point of 525 g of water containing 25.0 g of NaCl assuming a van’t Hoff factor of 1.85 Problem Solving Calculate the percent ionization of a 1.00 m aqueous acetic acid solution based on the following equilibrium: HC2H3O2 (aq) H+ (aq) + C2H3O2- (aq) Kf = 1.86C/m. The solution actually freezes at –1.90 C 12.74 The van’t Hoff factor for the solute in 0.118 m LiCl is 1.89. Calculate the freezing point of the solution. Why is this factor so much larger than that of NiSO 4? Problem Solving An experiment calls for the use of the dichromate ion in sulfuric acid as an oxidizing agent for propyl alcohol. The chief product is acetone which forms according to the following reaction: 3C3H8O + Na2Cr2O7 + 4 H2SO4 3C3H6O + Cr2(SO4)3 + Na2SO4 + 7 H2O the oxidizing agent is only available as sodium dichromate dihydrate. In theory how many grams of sodium dichromate dihydrate are needed to oxidize 21.4 g of isopropyl alcohol according to the balanced equation? continued The amount of acetone actually isolated was 12.4 g. Calculate the percentage yield of acetone The reaction produces a volatile byproduct. When a sample of it with a mass of 8.654 mg was burned in oxygen, it was converted into 22.368 mg of carbon dioxide and 10.655 mg of water. Assume any unaccounted for material is oxygen. Calculate the percentage composition of the byproduct and determine its empirical formula continued A solution prepared by dissolving 1.338 g of the byproduct in 115.0 g of benzene had a freezing point of 4.87C. Calculate the molecular mass of the byproduct and write its molecular formula. Osmosis Osmosis The semipermeable membrane should allow only the movement of solvent molecules. Therefore, solvent molecules move from pure solvent to solution. Osmosis The semipermeable membrane should allow only the movement of solvent molecules. Therefore, solvent molecules move from pure solvent to solution. Osmosis Equilibrium is reached when pressure produced by extra solution — the OSMOTIC PRESSURE, p p = cRT (where c is conc. in mol/L) counterbalances pressure of solvent molecules moving thru the membrane. 687320265 page 7 of 8 Dr. Myton, CH116 Osmosis Osmosis Osmotic Pressure Adding a solute increases osmotic pressure Osmosis Osmosis of solvent from one solution to another can continue until the solutions are ISOTONIC — they have the same concentration. Osmotic pressure in living systems: FIGURE 14.16 Problem solving 12.64 What is the osmotic pressure in torr of a 0.010 M aqueous solution of a molecular compound at 25C? 12.65 An aqueous solution of a compound with a very high molecular mass was prepared in a concentration of 2.0 g/L at 298 K. Its osmotic pressure was 0.021 torr. Calculate the molecular mass of the compound Osmosis Calculating a Molar Mass Dissolve 35.0 g of hemoglobin in enough water to make 1.00 L of solution. p measured to be 10.0 mm Hg at 25 °C C. Calc. molar mass of hemoglobin. Solution (a) Calc. p in atmospheres p = 10.0 mmHg • (1 atm / 760 mmHg) = 0.0132 atm (b) Calc. concentration Osmosis Calculating a Molar Mass Osmosis Calculating a Molar Mass Conc = 5.39 x 10-4 mol/L (c ) Calc. molar mass Molar mass = 35.0 g / 5.39 x 10-4 mol/L Molar mass = 65,100 g/mol Problem solving A 1.40 g sample of polyethylene, a common plastic, is dissolved in enough benzene to give exactly 100 mL of solution. The measured osmotic pressure of the solution is 1.86 mm Hg at 25 ºC. What is the average molecular mass of the polymer Surfactants 687320265 page 8 of 8 Dr. Myton, CH116