SPECTRA EXPERIMENT 17
advertisement

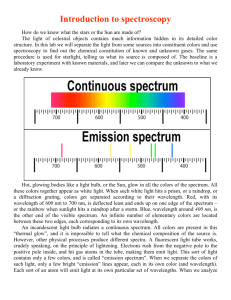
SPECTRA EXPERIMENT 17 PART I If available, observe flames from the following substances: Sodium compounds: NaCl, NaNO3 Lithium compounds: LiCl, LiNO3 Strontium compounds: SrCl2, Sr(NO3)2 Calcium compounds: CaCl2, Ca(NO3)2 Copper compounds: CuCl2, Cu(NO3)2 Q1 Are there any elements in the above group that have characteristic colors that can be recognized from the flame test? If so, name the element with the characteristic color and indicate the color. Q2 In this group are there any elements that you cannot distinguish by the flame test? If so, name them. GENERAL THEORY OF THE SPECTROSCOPE As shown in figure 1, part of a wide beam of light originating from a source A passes through a narrow slit B which defines a thin slice of light. The slice falls upon a diffraction grating C. The grating is a thin film on which there are ruled many parallel lines very close together. When the lines are parallel to slit B part of the light slice will be bent or diffracted at an angle as it passes through the grating. The amount of bending depends on two factors; d the distance between lines on the grating and the (wavelength or color) of the light hitting the grating. The exact relationship between , , and d for first order diffraction is: sin = d Thus as d increases, increases. Longer wavelength (red) light is bent more than shorter wavelength (violet) light. INCIDENT BEAM A B C figure 1 BENT BEAM If A is a source of white light (which contains all wavelengths) then an observer will see the white light spread out into a continuous spectrum of colors, with red at the largest angle. If A is a source of monochromatic light (which consists of a single wavelength) then an observer will see a single sharp line. During the course of this experiment you will observe both continuous and bright line spectra, the former characteristic of heated solid bodies, the latter isolated atoms in the gas phase being bombarded by electrons. For general information the wavelength associated with colors of visible light are listed in Table II, page 6. -1- PART II Observe an incandescent light source with your spectroscope. This will be a spectrum from a glowing solid. Q3 In your lab notebook draw a sketch of the spectrum making sure that you record the scale reading and colors associated with your own scale and spectroscope. Use the same spectroscope for the entire experiment. You should also note the position of each line you observe on a diagram like the one below: 700 600 500 400 BE SURE TO DRAW THE DIAGRAM TO SCALE. (No smaller than the sample) PART III Calibrate your spectroscope by observing the light from a fluorescent arc. All atoms and ions, when bombarded by enough electrons will emit light, which when passed through a spectroscope appears to an observer as a series of colored lines characteristic of the differences between the energy levels of the atoms or ions. In this experiment you will use a simple spectroscope capable of surprising accuracy t o study the bright line spectra of some simple ions and relate your observations to electronic transitions within the atoms. Look through the diffraction grating at the narrow end of the spectroscope pointing the slit towards the light source to be analyzed. The spectrum will appear on the right side of the slit below the scale where the wavelengths of the absorption or or emission lines can be read. The visibility of the spectrum can be improved by holding the hand around the narrow end of the spectroscope using the thumb and index finger to keep stray light from around the eye. The numbers on the scale represent 100 nm units. Looking through the spectroscope at a fluorescent lamp, all colors of the spectrum will be visible with three brighter lines- one violet line at 436 nm and one green line at 546 nm and one orange line at 605 nm.. If the lines are not exactly in place, the difference must be added or subtracted, respectfully, in all further observations. Q4 Sketch the spectrum of the fluorescent light as you did for the visible spectrum in part II. Record the color of the lines and their scale reading. Q5 Calculate the wavelength that corresponds to each of your scale readings for the fluorescent lines. A sample table is given below. (Include units on your values) (Scale reading = nm ) Line in fluorescent spectrum 1 2 color of line scale reading green violet ??? ??? -2- PART IV Observe the spectrum of some gaseous elements. Around the laboratory you will find discharge tubes that contain some gaseous elements. These tubes are connected to a high voltage supply so they will be energized to give off light. DO NOT TOUCH THE DISCHARGE TUBES, DO NOT CHANGE THE DISCHARGE TUBES. The reasons are they are very HOT, they have high electrical voltage, and if you break them they cost around $45.00. Q6 Observe each of the spectra and record the scale readings and the color of the various lines for each element. (Try to find 4 lines in the spectra of H2 if possible, three are easily distinguished.) PART V Observe the spectra of the unknown substances (X & Y). Q7 Record the scale readings of the unknown and diagram the spectra. Q8 For the H2 and He lights determine the wavelength that corresponds to each scale reading. Q9 Determine the percentage error for the experimental wavelength of He compared to literature values given in Table I. Do this for all lines in the He spectrum. Q10 What difference do you note in the spectra of tungsten as found in the incandescent lamp compared to the other elements. QUESTIONS 11-25 REFER TO THE HYDROGEN SPECTRA—All calculations from RED BLUE Q11 Calculate the frequencies of the hydrogen lines that you saw in your spectroscope. Convert nm to cm before determining frequency. (use page 5 of lab) Q12 Calculate the energy associated with each frequency. This is the energy difference in a transition of an electron from one energy level to another. Make sure to use Planck’s constant on pg 5 of lab. Q13 These spectral lines correspond to energy levels given off as electrons drop from a higher energy level to a lower energy level of -328 kj/mole. Calculate the value of the higher energy level from which the transition occurred in dropping to a lower energy level. The visible spectra occurs when transitions are made to the lower level of -328 kj/mole. (n=2) Energy difference (from Q12) = Higher energy level - lower energy level or E difference = Higher energy level - (-328 Kj/mole) Although the sign is negative it does not mean the energy is negative. This is merely convenience, a convention that has been chosen. -3- Review sections 4-1 and 4-2, pgs. 97-111 in your text. Q14 We can assign an integer, n, to each energy level that can exist. The energy level we choose as -328 kj in question 13 will be called n = 2. We will name each higher energy level with the next highest integer. Make a table relating the integer assigned to each energy with its energy level from Q13. Include n, n2, 1/n2, and the energy level value. n n2 1/n2 2 Energy level -328 Kj/mole_ 3 4 5 Q15 Plot the value of the energy level versus the integer n on a full page graph. Put n on the horizontal axis and E on the vertical axis with 0 being the top of the energy scale. Q16 From the shape of the graph in Q15 what type of relationship exists between n and E ? Q17 Plot the value of the energy level versus the value of n2. Q18 What type of relationship exists in Q17 ? Q19 Plot the value of the energy for the energy level versus the value of 1/n2. Be sure to convert the value of 1/n2 to decimals before plotting the graph. (example : 1/4 = .25 ) Q20 What type of relationship exists in Q19 ? -4- Q21 Draw a full page diagram like the one below. Note the value of n and the energy of the energy levels are to be included. The scale should be set up to range from -1312 Kj/mole as a minimum energy and 0 as the maximum energy. Make the diagram to scale. 5 4 Energy ( kj/mole) 3 n 2 -328 1 -1312 Q 22 Specify what the code letters were for the unknowns and what substances you determined to be present. Q23 What substance is present in fluorescent lights? (use your spectral data) EQUATIONS AND CONSTANTS TO BE USED E=h h = 39.8 x 10-14 kj.sec/ mole = c c = 3.0 x 1010 cm/ sec = wavelength = frequency in sec-1 nm x 1 x 10-7 cm = _______ cm %error = accepted value – experimental value x 100 accepted value -5- TABLE I --- REFERENCE SPECTRAL WAVELENGTHS A. Flame spectra (from the Handbook of Chemistry and Physics): in nm, (b) = position of band head, P = most persistent Barium: ------- 513 (b) ; 534 (b) ; 553 (b) P Calcium: ------ 554 (b) ; 618 (b) P ; 620 (b) P Lithium: ------ 610; 670 P Potassium: ---- 404; 404; 766 P; 769 P Sodium: ------ 589; 589 Strontium: ---- 606 (b) P ; 662 (b) ; 674 (b) B. Mercury arc: 623; 579; 577; 546; 496; 491; 435; 404 C. RARE GAS: He: 400; 460; 500; 585; 680 Ar: 489; 537; 544; 621 Ne: 585; 602; 640 Kr: 583; 564 __________________________________________________________________________________________ TABLE II -----THE VISIBLE SPECTRUM nm units Visible Spectrum----------------------------------------------------- 400--700 Violet (representative 4100), limits--------------------------- 400--424 Blue (representative 4700), limits--------------------------- 424--490 Green (representative 5200), limits--------------------------- 490--575 maximum visibility -------------------------------------------- 556 Yellow (representative 5800), limits--------------------------- 575--585 Orange (representative 6000), limits--------------------------- 585--647 Red (representative 6500), limits--------------------------- 647--700 Infrared, greater than ---------------------------------------------- 700 Ultraviolet, less than ---------------------------------------------- 400 -6-