Nuclear Reactions
advertisement

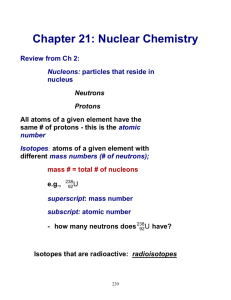
Nuclear Reactions Overview Nuclear reactions involve changes that take place within atomic nuclei (changing the element into another element or elements). Nuclear equations must be balanced with respect to atomic number and mass number. Example: 147 N + 10 n 146 C + 11 H Radioactivity A radioactive nucleus decomposes (decays) with the evolution of energy. Many nuclei exhibit natural radioactivity, others can be made in the lab by bombarding stable nuclei with high-energy particles such as neutrons. Modes of Radiactive Decay: (1) alpha (α) particle emission – an ordinary helium nucleus (42 He) is given off Ex: 23892 U --- 42He + 23490 Th (2) beta (β) particle emission – an electron is produced (0-1 e ) Ex: 23490 Th --- 0-1 e + 23491 Pa Beta emission results when a neutron converts to a proton and the beta particle- a fast moving electron in nuclei that have too many neutrons for stability. NOTE: The emission results in one more proton, no change in Mass Number because the neutron that decays becomes a proton. (3) gamma (γ) radiation emission – high energy photons, no particle mass. Gamma rays are omitted from nuclear equations (as it does not change atomic number or mass number). Artificially produced radioactive nuclei can exhibit α, β, or γ-emission, additionally they can exhibit: (1) positron emission – a particle identical to an electron with a +1 charge (01 e). Ex: 4019 K 01 e + 4018 Ar The atomic number decreases by one unit. Here a proton is converted to a neutron, when nuclei have too many protons for stability. (2) electron capture - an electron in the inner most energy level (n=1) falls into the nucleus – common with heavy nuclei because n=1 is closer to the nucleus Ex: 82 37 Rb + 0-1 e --- 8236 Kr Examples Promethium (Z = 61) is essentially nonexistent in nature; all of its isotopes are radioactive. Write balanced nuclear equations for the decomposition of : (a) Pm -142 by positron emission. (b) Pm-147 by beta emission. (c) Pm-150 by alpha emission. Bombardment Reactions More than 1500 isotopes have been prepared in the laboratory by bombardment reactions. In these reactions a stable nucleus is convert to one that is radioactive, which in turn decays to stable products. The bombarding particle can be: (1) neutron (at low energy produced by a fission reactor) Ex: 2713 Al + 10 n -- 2813 Al 28 13Al -- 2814Si + 0-1 e (beta emission) (2) a charged particle (electron, positron, α-particle, etc.) accelerated to high velocity in an electric and/or magnetic fields in a linear accelerator or cyclotron (circular accelerator) Ex: 2713 Al + 42 He --- 3015P + 10n 30 15P -- 3014Si + 01e (positron emission) An application of bombardment reactions is the preparation of transuranium (greater atomic # than uranium) elements (107 -112 and 114 in 1999 t1/2 = 30s). Applications I. Medical Applications A. Radiation therapy for cancer cells – radioactive isotopes (like Co-60) produce γ-rays that are focused on malignant cells Thyroid cancer can be treated by having a patient drink a solution of NaI (with radioactive iodide- 131I or 123I). The iodine moves to the thyroid and the radiation destroys malignant cells. B. Tracers use radioactive nuclei imaging for diagnosis – Positron emission tomography (PET) using C6H12O6 containing C-11, a positron emitter, to study the brain. Radioisotopes are used for test for anemia (5926 Fe), circulatory disorders (2411 Na), and lung disorders (13354 Xe) among many others. II. Commercial A. Smoke alarms- Smoke impedes the flow of air molecules ionized by Am-241 in an ionization chamber, causing current to drop and the alarm to sound. B. Food irradiation – Gamma rays are used to kill insects, larvae, and parasites (trichina). Radiation can also inhibit sprouting and kill microorganisms (E. coli). Shelflife can be extended by weeks or even months. C. Nuclear Fission -- The process by which a heavy nucleus is split into smaller nuclei, releasing energy in the process. Fission takes place when elements are bombarded with neutrons of high enough energy. 235 U 92 and 23994Pu are the most common isotopes used for the process. Example: More than 200 isotopes of 35 elements have been identified among the fission products of U-235. 90 1 0n 37Rb + 23592U ---- 72 + 14455Cs + 2 10n 87 30Zn 35Br + 14657La + 3 10n + 16062Sm + 4 10n The products are often radioisotopes can pose radiation hazards. Two to four neutrons are produced for every one consumed. These neutrons can be used to bring about the fission of more atoms (chain reaction). For nuclear fission to result in a chain rxn the sample must be large enough so that the neutrons are captured internally (too small and neutrons escape). The critical mass for U-235 is 1 to 10 kg. D. Nuclear Fusion— The process by which light nuclei combine with one another to form a heavier nucleus. The light isotopes (Hydrogen) for fusion are more common than the heavy isotopes need for fission (Uranium, Plutonium). Fusion processes have high activation energies (particles have to be accelerated to about 106 m/s to overcome repulsive forces, T = 109 0C). The Hydrogen bomb uses a fission reaction to generate sufficient heat to trigger a fusion reaction. Example: deuterium (hydrogen with one neutron). 2 1H + 3 1H -- 42He + 10n