Spectroscopy Lab
advertisement

Spectroscopy Lab
Atomic Spectra
When a low density gas is excited by an electric current it glows, it emits
light. When this light is sent through a prism or diffraction grating it is found to
consist of only a few colors. This set of colors is called the spectrum of the gas
(or the spectrum of the atom or molecule making up the gas). Every different
atom and molecule has a different spectrum. The same type of gas under the
same conditions will have exactly the same spectrum.
The spectra of atoms (and molecules) is a "fingerprint" that allows us to
identify the composition of things just from the specific colors of light they emit.
Spectroscopy – the study of spectra – has been used to determine the composition of unknown samples, other planets, distant stars, and more.
Hydrogen Spectrum
Unlike most elements, the spectrum of hydrogen has only a few colors
(wavelengths) and these form a simple pattern. The hydrogen wavelengths in the
visible part of the spectrum obey a formula called the Balmer series. With the
measurement of hydrogen wavelengths beyond the visible part of the spectrum,
the wavelengths in the hydrogen spectrum were all found to obey the formula
1
1
1
= Rn 2 –n 2
2
1
where n1, n2 = 1, 2, 3, … and n2>n1.
R = Rydberg constant = 1.097 x 107 m-1.
The Balmer series corresponds to n1=2 and n2 = 3, 4, 5, …
(Q1) Calculate the first three wavelengths in the Balmer series. Express in nm.
Put your answers on the data sheet that follows.
Bohr Theory
Why do atoms only emit certain colors? Neils Bohr created a model of the
atom to explain this. His model postulated that electrons move in circular orbits
about the nucleus and that only a few special orbits were stable. In classical
theory, orbiting electrons should lose energy and spiral down into the nucleus,
Bohr assumes this doesn't happen.
Electrons were allowed to instantly "jump" from one orbit to another but in
doing so they would have to either emit or absorb energy (photon) equal to the
difference in the energy level of the two orbits. The spectrum of an atom results
from the set of possible transitions between allowed orbits for its electrons.
The hydrogen spectrum is particularly simple because it has just one orbiting electron. Atoms with multiple electrons have more complex spectra due to
the interactions (repulsion) of electrons which shifts the energies of allowed orbits. The Balmer series of hydrogen corresponds to electron transitions from the
3rd, 4th, 5th, … allowed orbits down to the second allowed orbit.
Although the Bohr model works well to explain the hydrogen atom, it fails
to explain the full details of other spectra. The Bohr model has been replaced by
Quantum Mechanics. Quantum mechanics explains why only certain orbits are
allowed – basically electrons are waves and only standing wave patterns are
stable so only an orbital size equal to a certain number of wavelengths can exist.
Spectrometers
In principle, a spectrometer is the simplest of scientific instruments. Bend
a beam of light with a prism or diffraction grating. If the beam is composed of
more than one color of light, a spectrum is formed, since the various colors are
refracted or diffracted to different angles. Carefully measure the angle to which
each color of light is bent. The result is a spectral “fingerprint,” which carries a
wealth of information about the substance from which the light emanates.
The importance of the spectrometer as a scientific instrument is based on
a simple but crucial fact. Light is emitted or absorbed when an electron changes
its orbit within an individual atom. Because of this, the spectrometer is a powerful
tool for investigating the structure of atoms. It's also a powerful tool for
determining which atoms are present in a substance. Chemists use it to
determine the constituents of molecules, and astronomers use it to determine the
constituents of stars that are millions of light years away.
In its simplest form, a spectrometer is nothing more than a prism and a
protractor. However, because of the need for very sensitive detection and precise
measurement, a real spectrometer is a bit more complicated. Our spectrometers
consist of three basic components; a collimator, a diffracting element, and a
telescope. The light to be analyzed enters the collimator through a narrow slit
positioned at the focal point of the collimator lens. The light leaving the collimator
is therefore a thin, parallel beam, which ensures that all the light from the slit
strikes the diffracting element at the same angle of incidence.
Focusing the Spectrometer
1. While looking through the telescope, slide the eyepiece in and out until the
cross-hairs come into sharp focus. Loosen the graticule (see the figure on the
next page) lock ring, and rotate the graticule until one of the cross-hairs is
vertical. Retighten the lock ring and then refocus if necessary.
2. Focus the telescope at infinity. This is best accomplished by focusing on a
distant object (e.g.; out the window).
3. Check that the collimator slit is partially open (use the slit width adjust screw).
4. Align the telescope directly into the collimator.
5. Looking through the telescope, adjust the focus of the collimator and, if
necessary, the rotation of the telescope until the slit comes into sharp focus. Do
not change the focus of the telescope.
6. Tighten the telescope rotation lock-screw, then use the fine adjust knob to
align the vertical line of the graticule with the fixed edge of the slit. If the slit is not
vertical, loosen the slit lock ring, realign the slit, and retighten the lock ring. Adjust
the slit width for a clear, bright image. Measurements of the diffraction angle are
always made with the graticule line aligned along the fixed edge of the slit, so a
very narrow slit is not necessarily advantageous.
The spectrometer accessories case includes a magnifying glass to aid in
reading the vernier scale. Vernier scales are used in many types of precision
equipment so I want you to learn how to use such a scale.
From the position of the 0 (zero) tick mark on the upper scale, you determine the primary angular value. If the zero tick
mark is between, say, 266 1/2 and 267, then
you know the correct angle is somewhere
between 266.5° and 267°.
The vernier scale is used to determine
exactly what angle is correct between 266.5
and 267. There are 30 lines (numbered 0 to 30)
on the upper vernier scale, these lines are
spaced apart differently (slightly) than the lines
on the lower angular scale. Only one line on the
upper scale meets up exactly with a line on the
lower scale, which line tells you the amount
between 266.5 and 267 of the exact angle.
The vernier scale is using angles measured in degrees (°) and minutes (')
of arc. There are 60 minutes of arc in one degree of angle. So 266.5° is the same
as 266° 30'. The value of the line on the vernier scale (from 0 to 30) that aligns
best is the additional minutes of arc. If the 22' line on the vernier scale lined up
best, the angle would be 266° 30' + 22' = 266° 52' = 266° + 52/60 ° = 266.87°.
Look at the lower figure to the right to see if you understand how they
determined an angle of 155° 15'.
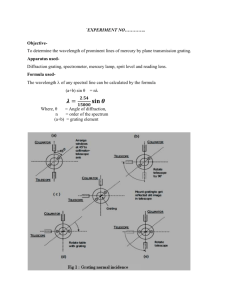
Diffraction Grating
IMPORTANT: The diffraction grating is a delicate component. Be careful not
to scratch the surface and return it to its protective box when not in use.
Insert the diffraction grating into the clips of the mount, make sure the
grating is horizontal. Place a hydrogen discharge tube approximately one centimeter from the collimator slit, turn on the tube. Adjust the slit width so the slit image is bright and sharp.
Use a vernier scale to measure the angular position of the central maximum. It doesn't matter which scale you use but you must use the same scale for
all your measurements. You can use this angle to determine if your grating is rotated or to re-align your spectrometer if it is bumped.
Rotate the spectrometer telescope until you see the spectral lines of hydrogen. You should clearly see a red line and a teal line plus a faint purple line
(the purple is closest to the central maximum then the teal then the red). You'll
probably have to turn off the classroom lights to see the purple line. It is possible
you'll see two purple lines, use only the one closer to the teal line. If you turn the
telescope further, you will see all the lines a second time, the second-order diffraction grating maxima.
Align the vertical cross-hair with the fixed edge of each spectral line image, record the angular position for each. The diffraction grating diffracts the incident light into identical spectra on either side of the undiffracted beam. Rotate the
telescope to the opposite side and record the angles for these spectral lines as
well. Measure angles for both the first and second order lines. Re-measure the
position of the central maximum at the end to make sure nothing changed.
For each spectral line in each order, calculate its angle from the central
maximum on each side, enter those values into the second table and average
those two values. If the angle to a line crosses the 360° mark, you must adjust
your calculation accordingly. For example, if the first-order red line is at angle
348.167° and the central maximum was at 13.083°, the angle to the line would be
24.916°.
(Q2) Based on the angles measured on each side, were the spectral lines at
symmetrical angles on each side of the central maximum? Were all the angles on
one side consistently larger than on the other? That could indicate a slightly
turned grating slide, but it won't affect the results unless severe.
The grating used has 300 lines per millimeter, this corresponds to a d
value of 3.33 x 10-3 mm = 3333 nm. Use the grating formula and your average
angles to determine the wavelength of each spectral line.
d sin = m
Use m=1 for the first-order lines and m=2 for the second-order lines.
(Q3) Compare your results to those calculated for the Balmer series in Q1.
Data Sheet for Spectroscopy Lab
Q1) n2 = 3
= _________________
n2 = 4
= _________________
n2 = 5
= _________________
Position of Central Maximum: _______°
_______'
Left
Side
= _________°
Right
Side
Positions
Angular
Order
Color
In °,'
m=1
Purple
Positions
Angular
Degrees
In °,'
Degrees
Teal
Red
m=2
Purple
Teal
Red
Order
Color
m=1
Purple
Teal
Red
m=2
Purple
Teal
Red
Left angle Right
angle
Average
Calculated
from
center
Angle
Wavelength
from
center