Synthesis, characteristics and analysis of Co(III) complexes of the
advertisement

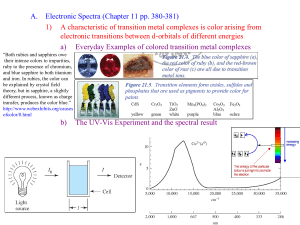
Synthesis, characteristics and analysis of Co(III) complexes of the type[Co(NH3)5X]n+ LORA Chem4415 Department of Chemistry, College of Science Sultan Qaboos University 2/04/2011 Objective: The purpose of this report is to show the synthesis of cobalt(III) complexes in the form of [Co(NH3)5X]n+, where X = Cl-, H2O, NO2, NH3 and ONO. In addition, the UV-Visible Spectra and IR spectroscopy of these complexes will be there with the discussion. Introduction: The elements in the periodic table are often divided into four categories: (1) main group elements, (2) transition metals, (3) lanthanides, and (4) actinides. A coordination complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bond to a central metal atom (or ion) by coordinate covalent bonds. All d-orbitals have the same energy (in spite of their different shapes and/or orientations) on a bare metal ion. However, some d-orbitals have different energies from the others in a metal complex. This is called d-orbital splitting. Cobalt (Co) is one of the transition metals that can be found in the first row of the transition metals and has a molar mass of 58.93 g/mol. Cobalt can form coordination complexes with different charges and each charge can have different properties from the other properties of the other charge. The chemistry of cobalt which forms complexes that contain either Co2+ or Co3+ ions. The electron configuration of a neutral cobalt atom is written as follows. Co: [Ar] 4s2 3d7 The discussion of the relative energies of the atomic orbitals suggests that the 4s orbital has a lower energy than the 3d orbitals. Thus, we might expect cobalt to lose electrons from the higher energy 3d orbitals, but this is not what is observed. The Co2+ and Co3+ ions have the following electron configurations. Co2+: [Ar] 3d7 Co3+: [Ar] 3d6 This report deals only with Cobalt(III) (Co3+) coordination compounds Co(ІІІ) has high value of LFSE and this effect on the kinetic internist. Almost all Co(ІІІ) complexes are octahedral geometry; also they can have high or low spin properties. They are diamagnetic compounds, so they are repelled by a magnetic field and that causes their weight to decrease. High spin (paramagnetic) Co(ІІІ) has high value of LFSE and this effect on the kinetic internist. In this report I will deal with the complexes [Co(NH3)5X]n+ (X = Cl-, H2O, NH3 and ONO-). Experimental: Materials: All chemicals and solvents were commercially available and used as received. CoCl2.6H2O and concentrated HCl(aq) were purchased from Oualigens whereas NH4Cl, NaNO2, 30 % H2O2 and activated charcoal were obtained from Aldrich, BDH, Fluka and SD’S respectively; also concentrated NH3(aq) was available. Physical measurements: Melting points were obtained using a 7665 Zubair melting point apparatus. Infrared spectra were recorded as KBr discs on a 7584 Muscat Private spectrophotometer in the range 2000/800 cm-1 and UV-visible spectra were measured using a 3357 shimadzu spectrophotometer in the range 250-900 nm. Synthesizes of the complexes: 1- Synthesis of [ Co (NH3)5Cl ] Cl2 CoCl2∙6H2O (2.0875 g, 8.778x10-3 mol) was dissolved in a mixture of concentrated NH3(aq) (5 mL), distilled water (2.5 mL), and solid ammonium chloride (5.0344 g, 0.094 mol) in a 100-ml conical flask to give a dark brown slurry. Slowly and with continuous stirring (30%) H2O2(aq) (1.3 mL) was added to form dark brown mixture which then heated for 20 minutes resulting in purple paste. On addition of (25 mL) of 3 M HCl(aq) the color of the mixture was change to be bright purple. Then the solution was heated to 55- 60 °C for 15 minutes in which a pink color solution was formed. Then, the product was filtered off on a Buchner funnel and washed with 2 M HCl(aq) (8 mL). Then 2 M NH3(aq) (75 mL) was added and heating the product to 60 °C before the solution was filtered and transferred to a 250-mL conical flask. After that concentrated HCl (75 mL) was added dropwise as 25-mL every 10 minutes with heating, causing effervescence and heat release. After the last addition, the heating was continued for another 20 minutes. Then the mixture was filtered and allowed to cool to room temperature. Subsequently, the dark purple solid product was washed thoroughly with 2 M HCl(aq) and then with ( 95% ) EtOH until there were no further traces of hydrochloric acid in the washing so, the product was dried in air for 2 – 3 days. Yield: 0.6633g (30.17%) melting point : (303.2-304.3) °C 2- Synthesis of [Co(NH3)6]Cl3 CoCl2.6H2O(s) (5.0139 g, 0.021073 mol) and NH4Cl(s) (3.3053 g, 0.061802 mol) were mixed together with (30 mL) of distilled water in a 250-mL conical flask to form a dark pink mixture. With stirring, then activated charcoal (1.0050 g) and concentrated NH3(aq) (45 mL) were added to give dark-brown solution. The resultant slurry was cooled to 5 0C in an ice-water bath to be more dense than before. It seems to be dark-brown color after the addition of (4 mL) of 30% H2O2(aq). Due to the heating for about 30 minutes the color of the solution was changed to red-brown which then cooled in an ice-water bath to be with 0 0C. The product with charcoal were collected under suction by using Buchner funnel. Then, to separate the product from the activated charcoal, the solid (product and charcoal) was dissolved in a hot water (40 mL) in a conical flask and concentrated HCl(aq) (1 mL) was added to get dark brown mixture. Recrystallisation is necessary to separate the product from the activated charcoal, so the mixture was heated to be 70 0C hot and then filtrated. The dark orange filtrate was cooled and HCl(aq) (1 mL) was added dropwise to be more dense mixture with bright orange color. Before collected the product by decantation, it is important to keep it cooling for about 30 minutes to form precipitate. The product was dried in oven with 70 0C overnight to be ready as the final product. Yield: 2.90785g (51.598%); melting point: (300.6-302.1)ºC. 3- Synthesis of [Co (NH3)5(ONO)]Cl2:(my colleague result) [Co(NH3)5Cl]Cl2(s) (1.0005g, 0.0052mol) was dissolved in a mixture of 20 mL of water and conc. NH3(aq) (3 mL) in a 100-mL conical flask. Then a solution with dark purple color was appeared. Thereafter, the solution was heated and stirred, continuously the solid dissolved given a dark solution. Then the solution was cooled to room temperature and then in the ice bath. HCl(aq) (2M) was added to neutralize the solution and it must not be basic. Thereafter, the solution was cooled to below 5oC. After NaNO2 (1.0015 g, 0.0145 mol) was added, H2O group was replaced by NO2. Then a mixture of 1:1 H2O(l) and conc. HCl(aq) was added. Then the color of the solution was changed to orange. After the mixture was cooled in an ice bath for one hour, an orange precipitate formed. Then the product was collected by using Buchner funnel and then was washed by ice-cold water. Finally, the product was dried to room temperature to give an orange powder of [Co(NH3)5(ONO)]Cl2. Yield: 0.9262 g (92.16%), melting point: (260 – 262) ºC. 4- Synthesis of [Co(NH3)3(NO2)]Cl2 : (my colleague result) A purple powder of [Co(NH3)5]Cl2 (1.0005 g, 3.990 mmol) was added to a mixture of concentrated NH3(aq) (3 mL) and H2O(l) (20 mL) in 100-mL conical flask to give a dark purple slurry. The resultant mixture was stirred continuously and heated to boiling for about 3 minutes to give a dark red solution.. Next, the solution was cooled to room temperature then in ice bath. After that, the solution was neutralized by the addition of 2 M HCl(aq) (5 mL) and the color started to be lighter then before. The condition of the experiment at this stage must not be basic, so pH papers were required to test the solution. Thereafter, NaNO2(s) (1.2508 g, 18.129 mmol) was added to the solution and then the solution was made slightly acidic by the addition of 2M HCl(aq) where the effervescence occurred and an orange slurry formed. Subsequently, the mixture was slowly heated to boiling for less than 1 minute to produce an orange-yellow solution. After that, the solution was cooled at room temperature then in ice bath. An orange slurry was formed immediately after the addition of concentrated HCl(aq) (5mL).At the end, the mixture was kept to cool in an ice bath for 1 hour. During that period, the yellow product started to precipitate. The solid then was filtered off and kept at room temperature for 1 weak to dry in air. Yield: 0.5149 g (49.4 %); melting point: (231-234) ºC. 5- Synthesis of [Co(NH3)5(H2O)]Cl2: [Co(NH3)5Cl2]Cl2 (1.0100 g, 4.03 x10-3 mol) was dissolved in 5 % NH3(aq) (15.15 mL) in a 100-mL conical flask to produce a purple solution. The solution was stirred and heated for about 15-30 minutes. Thereafter, the mixture was dissolved and a purple color solution was appeared. Then the mixture was cooled to 10 0C in an ice bath. After conc. HCl(aq) (2.7 mL) was added, a red slurry was formed. Then the mixture was cooled to about 3 0C. The product was filterd off using a Buchner funnel. Then an ice-cold ethanol (6.7 mL) was added to wash the product. Finally, the product was dried in the room temperature to give a red powder of [Co(NH3)5(H2O)]Cl2 . Yield: 0.3849 g (35.55 %); melting point: (301.8-303.8) 0C. Result and discussion: 1- Synthesis of [Co (NH3)5Cl] Cl2: The resulting purple solid was synthesized by several steps which explained below: CoCl2 .6 H 2O( s ) (2.0875 g , 8.7780 10 3mol ) Mixture NH 4Cl( s ) (5.0344 g , 0.0940 mol ) 28% NH 3( aq) (5ml ) H 2O( l ) (2.5 ml ) Dark brown solution 30% H 2Oaq 1.3 mL Dark red solution heating T 60 65 oC stirring (20 min) pink solution 3 M HClaq 25 mL heating (60 0C ) pink solid filteration 2 M HClaq (8 mL) pink solid 2 M NH 3aq (75 mL) heating (T 60 0C ) stirring pink solution filteration concentrated HCl( aq) (75 mL) heating ( T 60 650 C ) 40 min purple solution filteration cooling (T RT ) yield 30.17%, , melting po int : 303.2 304.3 0C The Co (II) was oxidized to Co (III) by using an oxidizing agent H2O2 that leads to fast redox reaction and can easily break the single bond. Also, it was added to generate a large excess of the NH3 ligands. The equations below show the half reactions (oxidation and reduction) in the addition of H2O2. 2[Co 2 ( aq) Co 3 ( aq) e ] 2e 2 H ( aq) H 2 O2 ( aq) 2 H 2 O( aq) ______________________________________ 2Co 2 ( aq) 2 H ( aq) H 2 O2 ( aq) 2Co 3 2 H 2 O(l ) The red color of the solution was appeared after adding H2O2 due to the aqua. The following equation shows the overall reaction after the addition of H2O2 : 2[Co 2 ( aq) NH 4 ( aq ) 4 NH 3 ( aq) 1 / 2 H 2 O( aq) [Co( NH 3 ) 5 H 2 O]( aq) ] In addition, to replace an aqua ligand from the complex HCl(aq) was added with chloro ligands as shown in this equation: [Co( NH3 )5 H 2O]3 ( s ) 3Cl ( aq) [Co( NH3 )5 Cl ]Cl2( s ) H 2O(l ) in which Cl- ions were responsible for the purple color of solution. So, the overall reaction of the final product was: 2CoCl2 6H 2O( s ) 2 NH 4Cl( s ) 8NH3( aq) H 2O2 ( aq) 2[Co( NH3 )5 Cl ]Cl2 ( aq) 14H 2O(l ) The complex was deposited as a purple solid in 30.17% yield. It is clear that that the yield was so low because of the losing of some product during filtration of the product, or when transferring or weighting the starting material. From the geometric structure of the final product below, it seems that the coordination number which is the secondary valence of the central atom is 6 and the primary valence is 3. Also, the valence of NH3 and Cl- both equal to 1. It clear that the complex is octahedral with 6 ligands ( 5 NH3, 1 Cl- ) and 2 Cl- as counter ions. Therefore the total ions from Blomstrand Chain Formula of the complex should be 3 (1 cation, 2 anions).But when I measured the electrical conductivity of the final product, the specific conductance was 0.37 which gives (370 Ω-1cm-1mol-1) molar conductivities that means the compound should has 4 ions and that is not true. The reason for that result was due to the ionized water which was used to prepare the 0.001 M solution of the compound so, the specific conductance become 0.28 (0.37-0.09) which gives (280 Ω-1cm-1mol-1) as the molar conductivity that the corresponding number of ions equal to 3. H3N Cl NH3 Co H3N Cl2 NH3 NH3 Figure 1: the structure of [Co (NH3)5Cl] Cl2 2- Synthesis of [Co(NH3)6]Cl3: The complex was synthesized in many steps beginning with CoCl2.6H2O in which the Co(II)(aq) ion was oxidized by using H2O2 as the following equations that show the Oxidation- Reduction reactions and the overall redox reaction. 2Co2 ( aq) 2Co( aq) 2e H 2 O2( aq) 2e 2 H ( aq) 2 H 2 O(l ) ــــــ ــــــــــ ــــــــــ ــــــــــ ــــــــــ ــــــــــ ــــــــــ ــــــــــ ــــــــــ Co2 ( aq) H 2O( aq) 2H ( aq) 2Co3 ( aq) 2H 2O Activated charcoal was used as catalyst. It is ability to act as a surface-active crystal, stimulate the reaction and to replace aqua in [Co(NH3)5(H2O)]Cl2 with NH3. The red color of the solution was due to the addition of H2O2(aq). In this case cool the solution on ice bath because 30% hydrogen peroxide is not stable, it's broke down to H2O and the cooling was to minimize the breaking. Filter the mixture and wash it with hot water were to remove any carbon there. On the other case the change of the color from red to pink because of the addition of HCl(aq) which was added two times, to neutralize the product in first case and to recrystallize the product in the second case. The complex was deposited as orange solid in 51.59% yield. It seems lower than what we expected. The reason for that was of the starting material CoCl2·6 H2O which contained some impurities and may be some of them was loosed during the filtration, transferring and weighting. The equation of the overall reaction is shown: activated 2CoCl2 .6 H 2 O( s ) 2 NH 4 Cl( s ) 10 NH 3( aq) H 2 O2( aq) charcoal 2[Co( NH 3 ) 6 ]Cl3( s ) 14 H 2 O(l ) From the geometric structure of final product below, it is clear that the complex is octahedral with 6 ligands ( 6 NH3) and 3 Cl- as the counter ions. Therefore the total ions from Blomstrand Chain Formula of the complex should be 4 (1 cation, 3 anions) and that what I got when I measured the conductivity of the final product. The geometric structure of the product is shown: H3N NH3 NH 3 Co H3N Cl3 NH3 NH3 Figure 2: the structure of [Co(NH3)6]Cl3 3- Synthesis of [Co(NH3)5(ONO)]Cl2 : The equations of producing pentaamminenitritocobalt(III) chlorate are: CoNH Cl Cl H O NH 3 5 2 s 2 CoNH H OCl 3 5 2 3 aq 3 aq l CoNH 3 5 H 2OCl3aq NaNO2aq CoNH 3 5 ONO Cl2 s Here sodium nitrite was used to replace H2O group by NO2 group. H3N ONO NH3 Co H3N Cl2 NH3 NH3 Figure 3 : the structure of [Co(NH3)5(ONO)]Cl2 Because the solution was slightly acidic in the synthesis of [Co(NH3)5(ONO)]Cl2(s) it converted to [Co(NH3)5(NO2)]Cl2(s) in this experiment due to failed on controlling the PH conditions. 4- Synthesis of [Co(NH3)5(NO2)]Cl2: The equations below show the reactions to get the final product: CoNH Cl Cl H O NH 3 5 2 s 2 CoNH H OCl 3 5 2 3 aq l 3 aq CoNH 3 5 H 2OCl3aq NaNO2aq CoNH 3 5 NO2 Cl2s H3N NO2 NH 3 Co H3N Cl2 NH3 NH3 Figure 4: the structure of [Co(NH3)5(NO2)]Cl2 The differences between [Co(NH3)5(ONO)]Cl2 and [Co(NH3)5(NO2)]Cl2 [Co(NH3)5(ONO)]Cl2 [Co(NH3)5(NO2)]Cl2 pH conditions Basic Slightly acidic Temperature Ice bath Heating to boiling 1g 1.25 g Conditions Amount of NaNO2 5- Synthesis of [Co(NH3)5(H2O)]Cl3: The reaction for getting pentaammineaquacobalt(III) chlorate is explained in the following equation: CoNH Cl Cl 3 5 2 aq NH 3aq HClaq CoNH 3 5 H 2OCl3 s Adding excess amount of water in this experiment was to replace the chloro ligand with aqua ligands. also, heating was used to dissolve the compounds and to evaporate any gas. From the geometric structure of the final product below, it seems that the coordination number which is the secondary valence of the central atom is 6 and the primary valence is 3. Also, the valence of NH3, H2O and Cl- all equal to 1. It is clear that the complex is octahedral with 6 ligands ( 5 NH3, 1 H2O) and 3 Cl- as counter ions. Therefore the total ions from Blomstrand Chain Formula of the complex should be 4 (1 cation, 3 anions). The specific conductance that I measured for the final product was 0.44 which gives (440 Ω-1cm-1mol-1) molar conductivities that means the compound should has 4 ions and that what we expected to get. H3N H2O NH 3 Co H3N Cl3 NH3 NH3 Figure 5: the structure of [Co(NH3)5(H2O)]Cl2 There are three ways of forming solid products as we find in the lab cooling the solution to crystallize the product, separating from the charcoal by filtration then recrystallized the product or evaporating the solution to get the product. Also, there are many ways to isolate the product even filtration by suction on a Buchner funnel or filtration by using a glass funnel. In addition, to dry the solid products several ways are possible such as dry it between filter papers, in an oven, in a desiccators or at room temperature. Melting Points of Products used for Identification the composition of the solid and to check the purity. The sources of the errors in the melting points are due to the impurities of the product or the overdue reading for the exact melting point. IR Spectroscopy of [Co(NH3)5X]n+ : IR spectroscopy is used to study the special vibrational modes of cobalt (III) complexes, to prove the presence of ammine ligands, the other substituted ligands and to distinguish between linkage isomers. There are three levels of spectroscopy which are electronic, vibrational and rotational. Vibrational spectroscopy has many modes that can be described as stretching, scissoring, rocking and twisting. The portion of the infrared region most useful for analysis of organic compounds is not immediately adjacent to the visible spectrum, but is that having a wavelength range from 2,500 to 16,000 nm. To measure the IR spectroscopy for a molecule: Compress the sample in KBr disk instead of compressed it in the nujol disk because nujol has IR absorption, so it will mix with the IR absorption of the sample. Use the IR spectrophotometer to obtain the IR spectrum for each complex. Complex Frequency of the broad (cm-1) Type of bond [Co(NH3)5Cl]Cl2 3300-3000 N-H 845-487 (less than 1000) Co-Cl 3300-3000 N-H 840-480 (less than 1000) Co-Cl 3300-3000 N-H 1430-1400 N=O 3300-3000 N-H 1110-1050 N-O 1430-1300 N=O [Co(NH3)6]Cl2 [Co(NH3)5(NO2)]Cl2 [Co(NH3)5(ONO)]Cl2 [Co(NH3)5(H2O)]Cl2 3600-3200 Table 1: the IR spectroscopy of the complexes * For all the graphs there are peaks in the range of 3000-3500 cm-1 which means that there is (N-H) functional group that is represented by ammine ligand (NH3) that is exist each complex. In this range the curve vibrates these indicates that there is more than one N-H bond. * Sharpe peaks indicates the presence of halides functional group which have wavelength less than 1000 cm-1. * In the graph of [Co(NH3)5(NO2)]Cl2 there is no peaks to indicate a single bond but have peaks to indicate the double bond in almost the same range for the double bond in the nitrito ligand. * The graph of nitrito has sharp peaks at 1110- 1050 cm-1 which point to the presence of a single N-O bond. [Co(NH3)5(NO2)]Cl2 and [Co(NH3)5(ONO)]Cl2 are isomers which they have the same molecular formula, but different structures. Nitro and nitrito complexes have similar formula but there are some differences between them. In the nitrito complex the double and single bond separated, so it has two peaks in the IR spectroscopy in the range of (1110-1050) and (1430-1300). While in the nitro complex the double bond delocalize between oxygen and nitrogen, so it has one peak in the IR spectroscopy in (1430-1400). O N O O N O Figure 6: Resonance structure of NO2- O N O Figure 7: Resonance Hybrid of ONO- NH3 H3N NH3 NH3 H3N Co H3N NH3 Co NH3 H3N O NH3 N N O Figure 8: structure of nitrito complex O O Figure 9: structure of nitro complex Figure 10: IR spectrum of [Co(NH3)6]Cl3 Figure 11: IR spectrum of [Co(NH3)5Cl]Cl3 Figure12: IR spectrum of [Co(NH3)5(NO2)]Cl3 Figure 13: IR spectrum of [Co(NH3)5(ONO)]Cl3 Figure 14: IR spectrum of [Co(NH3)5(H2O)]Cl3 Ultraviolet (UV)-Visible Spectroscopy To find the UV-visible spectra for these complexes. The mass of the product which gives 0.015 M was calculated first and dissolved completely with 25 mL of distilled water. To measure the absorptivity used a Hewlett Packard 8453 UV-visible spectrophotometer and from that we can calculate the molar absorptivity of complexes. This can be determined by using beer-Lambret equation: A lc Where: A = absorptivity, ε = molar Absorptivity, C = concentration, l = path length = 1 cm Complexes [Co (NH3)5Cl] Cl2 [Co(NH3)6]Cl3 [Co(NH3)5(H2O)]Cl2 Molarity Wavelength Absorptivity Molar (mol/L) (nm).λ 0.03297 0.028 0.001579 Color ∆◦ Absorptivity observed of the ε (cm-1mol-1 complexes L) (cm-1) 359 1.31 39.73 530 1.43 43.60 340 1.11 39.64 Dark 29412 475 1.36 48.57 orange 21053 319x103 Dark 19802 224x103 orange 28249 505 354 0.199 Table 2: experimental results of U-V and visible spectroscopy of [Co(NH3)5X]n+ Wavelength range (nm) Absorbed color Observed color < 400 ultraviolet --- 400-450 Violet yellow 450-490 Blue orange 490-550 Green red 550-580 Yellow violet 580-650 Orange blue 650-700 Red green > 700 infrared --- Table 3: The absorbed color of visible light and the observed color Bond type λ max/(Lmol-1 cm-1) Spin forbidden…………………….…. >1 Spin allowed, Laporte forbidden, (d-d)transition………………………… 20-100(Oh) Laporte allowed……………………… 250-1000(Td) Chargetransfer…………..………….. 1000-5000 Table 4: Types of Electronic Transition. purple 27855 18868 Figure 15: Electronic Absorption Spectra of [Co(NH3)5Cl]Cl2 at different concentration absorbance1(AU) absorbance2(AU) Concentration(mol/L) 1.31 1.43 0.3297 0.774 0.8312 0.2637 0.5838 0.6308 0.211 From the result in the table above, this complex has d-d transition and the splitting energy is medium because of the ligand which is σ donor only (NH3) and π donor ligand (Cl-) . Also, the observed color of the complex was purple which means that absorbed color was orange with high wavelength and low wave number. In the figure above it is clear that the graph has two peaks , according to Tanabe- Sugano diagram and the ground state of d6 of Co3+ low -spin which show that the complex has two transition 1A1g→ 1T1g and 1A1g → 1T2g. the first transition with high wavelength and the second one with the lower wavelength. Figure 16: Electronic Absorption Spectra of [Co(NH3)6]Cl3 at different concentration absorbance1(A.U) absorbance2(AU) concentration(M) at 340 nm at 475 nm 0.009 0.265486 0.341536 0.028 1.110545 1.358809 0.02 0.689055 0.851362 0.01 0.334083 0.401124 This is an octahedral complex with d-d transition and with 2 peaks like the previous complex. Figure17: Electronic Absorption Spectra [Co(NH3)5(H2O)]Cl3 at different concentration λ=505 λ=354 This complex contains π donor ligand (H2O) which gives high concentration Absorbance absorbance (M) molar absorptivity because of the charge transfer from ligand to metal. The Absorptivity of this 0.001579 0.199079514 0.278426 compound was very low comparing to the others and that occur due to our mistake for 0.000607 0.072341919 0.104077 0.000495 0.058890343 0.05889 choosing the best concentration for the solution. Summary This report concentrate on the synthesis and isolation of cobalt(III) complexes of the form [Co(NH3)5X]n+ where X = Cl-, NH3, NO2- and ONO-. Also it contains information about IR and UV-Visible spectroscopy. Moreover, it shows the difference between the nitro and nitrito complexes. References 1- F. A Cotton, G. Wilkinson and P. L. Gaus, Basic Inorganic Chemistry, John Wiley and Sons, 3th Ed,1998 2- G. L. Miessler and D. A. Tarr, Inorganic Chemistry, 2nd Ed, Prentice Hall, New Jersey, 1998. 3- S. S. Zumdahl, Chemistry, 3rd Edition, D. C. Heath and Company, Canada, 1993, pp. 111, 112, 949. 4- Wolfsburg, G., Inorganic Chemistry, Sausalito, CA: 2000, pp. 839-877 5- Kazua Nakamoto, John Wiley and sons, Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part A, 5th edition, New York, 1997, page (1-13) 6- Cotton, F. A.; Wilkinson, G.; Gaus, P. L., Basic Inorganic Chemistry, 3th Ed., New York: John Wiley & Sons, 1995, pp. 19-25. 7- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced Inorganic Chemistry, 6th Ed, John Wiley & Sons, New York, 1999. 8- 'Transition metals' http://www.chemicalelements.com/groups/transition.html Accessed in 20/11/2006. 9- http://en.wikipedia.org/wiki/Cobalt_hexamine 10 - http://www.smso.net/Cobalt(III)_hexammine_chloride