Experimental evaluation of the pressure and
advertisement

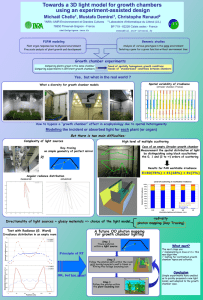
SUPPLEMENTARY MATERIAL Experimental evaluation of the pressure and temperature dependence of ion-induced nucleation Muhammad Miftahul Munir1,2, Asep Suhendi1, Takashi Ogi1, Ferry Iskandar1,2 and Kikuo Okuyama1,* 1 Department of Chemical Engineering, Hiroshima University, Hiroshima 739-8527, Japan 2 Department of Physics, Institut Teknologi Bandung, Bandung 40132, Indonesia * Author to whom correspondence should be addressed. Electronic mail: okuyama@hiroshima-u.ac.jp 1 Controlled RH System In order to study the pressure and temperature dependence of ion-induced nucleation, it is necessary to control the RH in the ionization chamber rather than control the H2O content (in ppm) in the mixing chamber. Fig. 1 shows a schematic diagram of a constant RH control system. T1, P1, TD, S, T2, and P2 are the mixing chamber temperature, pressure, dew-point sensor, SO2 sensor, ionization chamber temperature and pressure, respectively. Relative humidity (RH) data in the ionization chamber were calculated based on T1, P1, TD, T2 and P2. Relative humidity (RH) is defined as follows1: RH (%) 100 e es (S1) where RH, e and es are the relative humidity, actual water vapor pressure and saturation water vapor, respectively. The dew point temperature is the temperature to which the air must be cooled in order to be saturated with water. Therefore, water vapor pressure (e) is the saturation of water vapor at dew point temperature (td): e e s t d (S2) The Magnus formula describes the approximation between water vapor pressure (es) and temperature (t): 1-3 At e s C exp Bt (S3) Alduchov and Eskridge proposed the values A, B, and C of 17.625, 243.04 C and 610.94 Pa, respectively.3 By substituting equation (S3) for (S2), we obtain the following: At d e C exp B td (S4) Substituting equation (S3) and (S4) for equation (S1), we get the relationship between RH, 2 dew point and temperature as follows: At d At RH 100 exp B td B t (S5) Relative humidity in the mixing chamber (RH1) can be calculated as follows: At d At1 RH 1 100 exp B t d B t1 (S6) When gas-containing water vapor moves from chamber 1 (P1,T1) to chamber 2 (P2,T2), the physical properties of the gas mixture change. If the ratio of P2/P1 is defined as X, the water vapor pressure in chamber 2 can be expressed as follows: e2 Xe1 (S7) Where e1 is as follows: At d 1 e1 C exp B t d1 (S8) From equation (S8) we derive the relationship for water vapor pressure in chamber 2 as follows: At d 2 e2 C exp B td 2 (S9) Substituting equation (S7) for (S9) we get the following: td 2 Xe B ln 1 C Xe A ln 1 C (S10) Relative humidity in the ionization chamber (RH2) can be calculated as follows: At d 2 At 2 RH 2 100 exp B td 2 B t2 (S11) Based on equations (S8) to (S11), RH in the ionization chamber depends on the values of T1, T2, P1, P2 and TD. With a constant flow rate of MFC3, water content, measured by TD, was 3 controlled by the flow rate of MFC1 and MFC2. Based on this relationship, the control system was designed to provide a constant RH in the ionization chamber by adjusting the flow rate of MFC1 and MFC2 in response to the change in the dew point (TD), T1, T2, P1 and P2. The control system was realized by a program saved in the computer, while the desired value of RH was an input to the program. The proportional-integral-derivative (PID) control action, a program saved in the computer, was employed to attain higher reliability of the control system.4 References 1 M. G. Lawrence, Bull. Am. Meteorol. Soc. 86 (2), 225 (2005). 2 C. J. Gibbins, Ann. Geophys. Atmos. Hydrosph. Space Sci. 8 (12), 859 (1990). 3 O. A. Alduchov and R. E. Eskridge, J. Appl. Meteorol. 35 (4), 601 (1996). 4 M. M. Munir, F. Iskandar, Khairurrijal, and K. Okuyama, Rev. Sci. Instrum. 80 (2) (2009); M. M. Munir, F. Iskandar, Khairurrijal, and K. Okuyama, Rev. Sci. Instrum. 79 (9) (2008). 4 Figure S1. The negative (a) and positive (b) ions mobility distribution dependence on flow rate under Soft-Xray irradiation. The negative (c) and positive (d) ions mobility dependence on flow rate under radioactive irradiation. 5 Figure S2. (a) The negative ion mobility distribution dependence on pressure in the range of 600-980 hPa. The chamber temperature and SO2 concentration were 20 °C and 0 ppm, respectively. 6 Figure S3. Mechanisms of particle formation by Soft-Xray irradiation in SO2/ H2O/N2 mixtures. 7 Figure S4. (a) The dependence of Negative ion and charged particle mobility distribution on pressure. (b) The calculated mean particle diameter of positively and negatively charged particles under different levels of pressure. The chamber temperature, H2O and SO2 concentration were 20 °C, 4000 and 6.7 ppm, respectively. 8 Figure S5. (a) The dependence of Negative ion and charged particle mobility distribution on temperature. (b)The calculated mean particle diameter of positively and negatively charged particles as a function of temperature. The chamber pressure H2O and SO2 concentration were 980 hPa, 4000 and 6.7 ppm, respectively. 9 Figure S6. The calculated mean particle diameter of positively and negatively charged particles as a function of pressure. The chamber temperature, RH and SO2 concentration were kept at 10 °C, 60% and 0.65 ppm, respectively. 10 Figure S7. The calculated mean particle diameter of positively and negatively charged particles as a function of temperature. The RH and SO2 concentration were kept at 70% and 0.65 ppm, respectively. 11 Figure S8. The relationship between RH and the calculated mean particle diameter of positively and negatively charged particles. The pressure, temperature and SO2 concentration were kept at 980 hPa, 10 °C and 0.65 ppm, respectively. 12 Figure S9. The effect of SO2 concentration on the electrical mobility distribution of positive ions and charged particles. The pressure, temperature and RH were kept at 980 hPa, 10 °C and 70%, respectively. When SO2 was not present, only the ion was visible. 13 Figure S10. The effect of SO2 concentration on the mean particle diameter of positively and negatively charged particles. The pressure, temperature and RH were kept at 980 hPa, 10 °C and 70%, respectively. 14 Figure S11. The effect of residence time on the peak concentration of positively and negatively charged particles. The pressure, temperature and RH were kept at 980 hPa, 10 °C and 70%, respectively. 15