Word Version - People.vcu.edu
advertisement

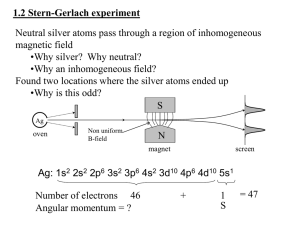
NMR Spectroscopy For background information, read Experiment 42 and 43 in Shoemaker et al. and Hoskins, L. C. J. Chem. Ed. 1991, 68, 83.You should focus on the theory of NMR, shielding, spin-spin splitting, and the experimental methods of NMR (FT vs. continuous NMR). Also, the physical chemistry textbook by Atkins has an excellent discussion of NMR. Introduction This experiment has two parts; they can be done in either order. The first part follows Experiment 42 in Shoemaker et al.: you use NMR spectroscopy to determine the equilibrium constant of the keto-enol equilibrium of acetoacetone. The second part follows the experiment described in Hoskins: you determine the splitting constant J of 2-chloroacrylonitrile and 2-bromothiazole. Spin Angular momentum is a measurable physical property of an object that results from rotation about a point (like the Earth around the sun) or rotation of the object about its center-of-mass (like the Earth's daily spinning). The angular momentum of small particles such as electrons can only have integer values, (i.e., 0, 1, 2...) starting with zero. This is the orbital angular momentum resulting from the motion of the electron around the nucleus. In addition, some particles may have half-integral angular momentum. This has been observed experimentally and derived from quantum mechanics. This half-integral angular momentum is an intrinsic property of the particle (in the same way that mass and charge are intrinsic properties). The halfintegral angular momentum is called spin, in analogy with the rotation of the Earth about its axis, but the term spin is only a metaphor. The electron is not really spinning. (Spin angular momentum can have integral or half-integral values, i.e., 0, 1/2, 1, 3/2, ...) 1H-NMR depends on the spin of the H nucleus. The H atom nucleus has spin 1/2. The angular momentum of the world of quantum mechanics obeys a set of rules different from the rules of the macroscopic world. The first rule has been described above: the angular momentum cannot have any value, but can only have certain specific or quantized values. The second rule concerns the vector properties of the angular momentum. Angular momentum has a magnitude (say 1/2 for a proton) and a direction. The rules say that both the magnitude and the direction of the angular momentum vector cannot be known simultaneously. For the proton, the magnitude is known and the projection of the vector on the z-axis is known. The x- and yprojections of the vector are unknown. The spin vectors are said to precess around a cone centered on the z-axis (see the picture in Shoemaker, page 457). The projection of the proton's spin angular momentum can be +1/2 () or -1/2 (). The frequency at which the vectors precess about the z-axis is called the Larmor frequency. The third rule concerns the way the spins of nearby particles interact or "couple" (when two vectors couple, they add or subtract). Just as the magnitude of the spin angular momentum vector can only have certain fixed values, and the projection of the vector can only have certain fixed values, the coupled vectors can only have certain fixed values. This coupling scheme is called the Clebsch-Gordon series. 1 H-NMR spectroscopy arises from the two projections of the proton's spin on the z-axis. In a magnetic field, these two projections have different energies. The energy difference depends on the magnetic field strength and is related to the Larmor frequency. For a 12 Tesla magnetic field, the energy difference is just 0.2 J mol1. According to the Boltzmann distribution, more particles will occupy the lower energy state than the higher energy state. In essence, an NMR spectrum records the energy difference between these two states. A compound is placed in a magnetic field inside the spectrometer. The protons occupy either the lower or higher energy state. When doing continuous NMR, radiofrequency radiation is applied, inducing a transition from low to high energy (a spin flip) if the frequency matches the Larmor frequency. Protons in different chemical environments will have different energy separations because they feel a slightly different magnetic field. The peaks in the NMR spectrum correspond to these energy differences. The position of these peaks is called the chemical shift. See Shoemaker et al. for discussion of these chemical shifts. Individual peaks in an NMR spectrum are often split into multiplets - doublets, triplets or quadruplets. This splitting arises from the coupling of the spin angular momentum vectors. The coupling strength, called the coupling constant J, determines the extent to which the chemical shift is affected by the splitting. J is measured from the spectrum; it is the difference between the peaks in the multiplets (see Figure 2 in Shoemaker Experiment 42). For protons attached to adjacent C atoms, the coupling constant J is much smaller than the chemical shift. The coupling constant for protons attached to C atoms separated by one or more carbons is two small to be observed. The splitting pattern is outlined in the table below. this group splits -CH-CH2-CH3- proton(s) of adjacent C atom into two energy levels three energy levels four energy levels giving a multiplet doublet triplet quartet You will record NMR spectra of 2-chloroacrylonitrile, CH2=CCl(CN) and 2-bromothiazole, both compounds with 2 chemically different protons which have different chemical shifts. Because the protons are attached to the same carbon, the coupling constant J for the two protons is large and similar in magnitude to the chemical shift. Thus, rather than observing two peaks each split into a doublet, we observe a broadly spaced quartet. Procedure for the Spin Part of the Lab Record the NMR spectra of 2-chloroacrylonitrile and 2-bromothiazole using the 60 MHz spectrometer. The solvent for each is CDCl3, with TMS (tetramethylsilane) included as the reference. (Why TMS?) Integrate the resulting peaks three times. Repeat, so that you have three spectra of each compound. Repeat using the 300 MHz spectrometer in place of the 60 MHz spectrometer. Data Analysis: 1. For each compound make a table of the four intensities (measured from the spectra), the four frequencies (measured from the spectra), n0, D, J, and n0(1) andn0(2) (measured from the spectra), the four energies (calculated), and q (calculated). Use average values and report uncertainties (one standard deviation). Repeat for the 300 MHz spectra. (In other words, your table will have four columns, two for each compound.) Why do some, but not all, of the numbers differ for the two different spectrometers? Why do they differ for the two different compounds? 2. Make an energy diagram showing the relative positions of the four energy levels based on your results. 2. Use the four energy formulas to calculate the four transition frequency formulas. The frequencies will have the form n0 plus or minus a term that depends on D and J. Now arrange these four frequencies in order from lowest to highest. 3. Now, subtract the two highest frequencies from each other and the two lowest frequencies from each other. You should get the same answer for each subtraction. 4. Sketch a rough picture showing the four peaks. On this picture indicate n0, D, J, and n0(1) andn0(2). [Hint: D and J are differences. n0, n0(1) andn0(2) are absolute positions]. 5. Compare your results to those of Hoskins. Procedure for the Keto-Enol Equilibrium Part of the Lab: Follow Shoemaker et al. Experiment 42. Results and Discussion for the Keto-Enol Equilibrium Part of the Lab.: Follow Shoemaker et al. Experiment 42.