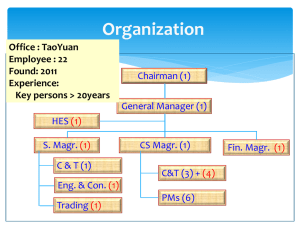
1.2: Definition of Clinical Waste
advertisement

DRAFT FOR COMMENT Environment Agency Technical guidance on Clinical Waste management facilities Version 3.0 December 2006 Comments should be sent to Peter Duffy by 8th January 2007: peter.duffy@environment-agency.gov.uk. 1 of 48 DRAFT FOR COMMENT Contents 1 Introduction 2 Permitted Wastes 3 Waste Acceptance, Labelling and Tracking 4 Waste Storage 5 Validation 6 Emissions Monitoring Appendix A : Site Commissioning Methodology Appendix B : Routine Efficacy Monitoring Appendix C : Emissions Monitoring and Benchmarks Appendix D : Modern Regulation 2 of 48 DRAFT FOR COMMENT 1: Introduction This guidance is an update to version 2.5 of the Technical guidance on Clinical Waste Management facilities. It provides additional information on the principles to be applied to Alternative Treatment processes (i.e. thermal and chemical) for rendering safe infectious healthcare waste (Clinical Waste). It does not cover incineration. This guidance is intended to incorporate the technical issues recently consulted upon in the supplement to Sector Guidance Note IPPC S5.06. The overarching principles of Waste Management Licensing should be applied to the Alternative Treatment processes that require a Waste Management Licence, however this guidance identifies some additional and alternative standards to be applied to Alternative Treatment plants. 1.1 Application of this document. The document is non-statutory guidance applicable to activities that fall within Waste Management Licensing Legislation and does not apply to sites regulated under a Pollution Prevention and Control Permit. Sections 2 and 5, Appendices A,B, and D apply only to treatment. Sections 1, 3 , 4 and 6, and Appendix C apply to both treatment and transfer. 1.2: Definition of Clinical Waste Clinical Waste is defined in the Controlled Waste Regulations 1992 as: “(a) any waste which consists wholly or partly of human or animal tissue, blood or other body fluids, excretions, drugs or other pharmaceutical products, swabs or dressings, or syringes, needles or other sharp instruments, being waste which unless rendered safe may prove hazardous to any person coming into contact with it; and (b) any other waste arising from medical, nursing, dental, veterinary, pharmaceutical or similar practice, investigation, treatment, care, teaching or research, or the collection of blood for transfusion, being waste which may cause infection to any person coming into contact with it” Healthcare waste is a waste classified under Chapter 18 of the List of Wastes, which is waste from natal care, diagnosis, treatment or prevention of disease in humans/animals. Examples of healthcare waste include: infectious waste; laboratory cultures; anatomical waste and animal carcasses (infectious/non-infectious); sharps waste (radioactive, cytotoxic and cytostatic, medicinally contaminated, body fluid contaminated); medicinal waste (cytotoxic and cytostatic, other); chemicals (occur both as ‘laboratory smalls’, and diagnostic reagents/sample preservatives in healthcare waste streams); offensive waste (non-infectious); amalgam; 3 of 48 DRAFT FOR COMMENT The relationship between the definitions of healthcare waste and clinical waste is as follows: (i) Healthcare waste includes wastes that are not hazardous, for example noninfectious hygiene wastes from patients. This waste would NOT be classed as clinical waste. (ii) Clinical waste can be produced from activities other than those included in Chapter 18 of the List of Wastes e.g. from body piercing, or arising as drug litter. This waste would NOT be classed as healthcare waste (iii) Healthcare wastes that present a hazard are also clinical wastes. With the introduction of the Hazardous Waste Regulations, the List of Wastes Regulations, the reduction in incineration capacity, and the introduction of nonincineration technologies, it is therefore now necessary to consider clinical waste in terms of: hazardous and non-hazardous waste, and List of Wastes classification, and appropriate disposal option (incineration / alternative treatment) rather than as the broad A-E groups indicated in the Safe Disposal of Clinical Waste 1999. Adequate source segregation should reduce the amount of healthcare waste that is classified as clinical waste, and therefore the quantity of healthcare waste that requires rendering safe. Technical Guidance WM2 provides the guidance on the definition and classification of hazardous waste. 1.3 Definition of Alternative Treatment Alternative Treatment includes treatment by heat, chemicals and irradiation in order to render clinical waste safe. At present, this mostly relates to treating infectious clinical waste to render it safe by disinfecting it. Thermal treatment uses heat to inactivate pathogenic micro-organisms. Heat treatment can be broadly divided into two groups. 1) ‘Moist’ heat processes that generate steam, including autoclaves, steam augers, and some microwaves 2) ‘Dry’ heat processes utilising electricity, hot-oil and some microwaves, which rely partially on moisture already within the waste. Chemical treatment utilises disinfectants (e.g. sodium hypochlorite, chlorine dioxide, peracetic acid, glutaraldehyde, calcium oxide or quaternary ammonium compounds). Treatment of clinical waste by gamma irradiation has also been used. Emerging technologies, for example hot alkaline hydrolysis, may have scope to treat other hazardous components of clinical waste, beyond just disinfection. Potentially this provides for a broader range of waste types that could be treated. Explanation and discussion of various technologies is beyond the scope of this document. Further information can be found from the following sources 4 of 48 DRAFT FOR COMMENT - Healthcare without harm, Non-incineration Medical Waste Treatment technologies, http://www.noharm.org/europe/medicalwaste/nonincineration World Health Organisation, Safe management of Wastes from Healthcare Activities. http://www.healthcarewaste.org/en/documents.html?id=1 These documents are presented as information resources only. This guidance document is considered to take precedence over these for the purpose of the regulation of clinical waste in England and Wales. 5 of 48 DRAFT FOR COMMENT 2: Permitted Wastes 2.1: Definition of Rendering Safe Rendering safe is defined in the Department of Health document ‘Safe management of healthcare waste’ as treatment that: a. for infectious waste – has demonstrated the ability to reduce the number of organisms present in the waste to a level that no additional precautions are needed to protect workers or the public against infection by the waste; b. for anatomical waste – destroys any human tissue, organ or body part so that it is ruined, torn apart, or mutilated through processes such as thermal treatment, melting, shredding, grinding, tearing, or breaking such that it is no longer generally recognisable; c. for any clinical waste – renders any syringes, needles or any other equipment or item unusable and no longer in their original shape and form; d. for medicinal waste – destroys the component chemicals. 2.2: Wastes Suitable for Alternative Treatment (Disinfection) The purpose of Alternative Treatment (Disinfection) is to render infectious waste safe. Therefore the wastes to be treated should have properties that require rendering safe and have properties capable of being rendered safe by Alternative Treatment. If other wastes are to be treated using Alternative Treatment the reasons for treating this waste should be fully justified. The wastes listed in Table A2.1 below are identified in the European Waste Catalogue (EWC) as hazardous because they are infectious (Hazardous Property H9). Alternative Treatment should be restricted to these wastes unless the Licence holder can justify the treatment of other wastes. Guidance on how to determine if a waste falls within either of these codes is given in Appendix C of WM2: C9 Assessment of Hazard H9: Infectious. Table 2.2 – Wastes Suitable for Alternative Treatment EWC Code Description of Code Description 18 01 Waste from natal care, diagnosis, treatment or prevention of disease in humans 18 01 03* Waste whose collection and disposal is subject to special requirements in order to prevent infection 18 02 Waste from research, diagnosis, treatment or prevention of disease involving animals 18 02 02* Wastes whose collection and disposal is subject to special requirements in order to prevent infection. 20 01 Separately collected fractions (except 15 01) 20 01 99 Other fractions not otherwise specified (Separately collected fractions of municipal clinical waste whose collection and disposal is subject to special requirements in order to prevent infection. This may include waste from tattoo parlours, body piercing and blood contaminated clothing.) 6 of 48 DRAFT FOR COMMENT Wastes within these codes are infectious and require rendering safe. However, some wastes that may fall within these codes are not suitable for Alternative Treatment (disinfection) and some of these are discussed below. 2.3: Wastes Unsuitable for Alternative Treatment The following wastes are specifically excluded from alternative treatments, even if they fall within the codes identified in 2.2. Waste Management Licences should specifically exclude the following wastes from the treatment process: Table 2.3– Wastes Unsuitable for Alternative Treatment Description Reason Pharmaceutical waste in any form or Disinfection technologies have no container (particularly sharps boxes), recognised action against including medicinally contaminated pharmaceutical molecules. syringes. Cytotoxic and cytostatic contaminated waste. Anatomical waste and carcasses in any The Department of Health, document form ‘Safer management of healthcare waste’ states that ‘the treatment of anatomical waste requires that the waste be rendered unrecognisable in suitable licensed facilities, which at this time means incineration.’ All microbiological cultures from any See note below on standards for source and any potentially infected treatment of certain Biohazardous waste from pathology departments and wastes. other clinical or research laboratories (unless autoclaved before leaving the Guidance should be sought from the site of production). Health and Safety Executive before such wastes are moved (in untreated form) Any waste from containment level 3 from the premises of production. laboratories Any waste contaminated with UN transport Category A or ACDP hazard Group 4 pathogens Sharps may only be subject to Alternative Treatment where the following criteria have been met: No sharps or other material contaminated with cytotoxic and cytostatic medicines are present in the sharps box. No pharmaceutical waste is present in the box. The syringe body is considered to be a pharmaceutical waste when contaminated with, or containing residual quantities of medicines (some pharmaceutical waste, and manufactured pre-filled syringes are both sharps and pharmaceutical waste, and are therefore not suitable for alternative treatment). This means that medicinally contaminated syringes are not suitable for alternative treatment and must not be introduced into these processes. The Environment Agency no longer issues licenses on the basis of ‘fully discharged’ syringes or sharps for the following reasons:- 7 of 48 DRAFT FOR COMMENT This position was adopted solely for the purposes of consignment under the Special Waste Regulations 1996. These regulations have now been revoked, and the position has not been carried forward. Audits of healthcare producers in England and Wales has confirmed that waste producers rarely produce and segregate such waste, and where they do other issues listed here are highlighted. This may encourage secondary handling of syringes resulting in an increased risk of needlestick injuries in producer premises. This may encourage discharge of active pharmaceuticals to foul sewer that may result in harm to the environment or human health. License conditions relating to this term are difficult to enforce, giving rise to significant potential of inappropriate disposal of waste pharmaceuticals. Certain Biohazardous waste represent either a heightened risk, require a higher level of treatment, are categorised as a Category A waste for transport, or are subject to guidance from the Health and Safety Executive with respect to treatment on the site of production. The following additional criteria apply to their acceptability at alternative treatment (disinfection) facilities No wastes containing ACDP 4 pathogens are suitable for treatment No waste containing other listed unsuitable wastes are suitable for treatment. Processes employing physical treatment (maceration/shredding) of untreated wastes are unsuitable. A higher level of treatment (STAATT level IV criteria) is required for such wastes. Such wastes should not routinely leave the premises of production in untreated form, and should only arrive at an alternative treatment facility as a ‘one off’ where treatment at the site of production has temporarily broken down. 2.4: Other waste streams. Treatment for Pharmaceutical Wastes, and materials contaminated with pharmaceuticals, requires that all pharmaceutically active substances present in the waste, both hazardous and non-hazardous, should be destroyed during treatment. At present, high temperature incineration is the primary method of achieving this. It is possible that future alternative technologies, for example alkaline hydrolysis, may demonstrate treatment efficacy in this Area. In general this would require laboratory trials to demonstrate that process parameters are theoretically able to treat a very broad range of representative pharmaceutical molecules before testing protocols for device based trials could be considered. The disposal options for Anatomical waste are currently restricted to Incineration for ethical reasons. It is not considered appropriate or acceptable to shred and thermally/chemically treat anatomical waste. Novel technologies like alkaline hydrolysis that dissolve, and therefore utterly destroy, the tissue leaving a ‘bone shadow’ equivalent to incinerator ash may be applicable for such wastes. Treatment of non-infectious waste. Where the waste does not possess the hazardous property H9 Infectious, there it is unlikely that there is any benefit in subjecting such waste to a process that is designed to reduce infectivity. Licences should not be issued for treatment of such wastes unless the licence holder provides justification. Issues to consider Non-infectious wastes are likely to have a high moisture content (high specific heat capacity) or high organic content which can present additional and 8 of 48 DRAFT FOR COMMENT significant problems for the thermal and chemical treatment process. Validation protocols would therefore have to include a specific high organic challenge load. The mixing of hazardous waste with non-hazardous waste is contrary to the principles of the Hazardous Waste Directive. Licences should not be issued that allow mixing of clinical (infectious) waste with non-infectious waste during the treatment process. Is the waste being incorrectly described as non-hazardous/non-infectious ? For example clinical waste is hazardous waste with only two exceptions; where is a non-cytotoxic and cytostatic medicine, or where it is infectious clinical waste that arises as a separately collected municipal fraction and is therefore classified by the List of Wastes as non-hazardous under 20 01 99. An example of where treatment of non-infectious waste, separately from infectious waste, may be appropriate is Where the licence holder can demonstrate that treatment of non-infectious waste is necessary to reduce the number of non-pathogenic microorganisms present in the waste to facilitate its recycling. Such treatment may be justified as part of an authorisation of a genuine recycling or recovery operation. 9 of 48 DRAFT FOR COMMENT 3 : Waste Acceptance, Labelling and Tracking In developing the guidance the following have been considered: The examination of healthcare wastes by the carrier or consignee is severely limited by health and safety concerns. Producer segregation is often poor resulting in a mixed waste Paperwork descriptions have often historically been completed by the carrier, and frequently contains insufficient information for subsequent holders to discharge their duty of care. Particularly where waste passes through intermediary destinations and new paperwork is generated. The composition of the waste accepted at the site must be determined by either:1. Producer Audit - The Safe Management of Healthcare Wastes identifies the role of robust producer audit. The Environment Agency considers that the robust producer audits (as specified in 3.1) are the safest and most effective means of ensuring that the waste facility has sufficient information to conduct appropriate waste acceptance procedures in conjunction with the more cursory checks specified in 3.2. This section provided guidance on this option. OR 2. Waste Composition Analysis - the licence holder could in theory inspect the contents of all waste containers (sharps boxes, rigid bins, yellow bags etc) to confirm that the contents comply with the permitted wastes. Such on site audit facilities have previously been authorised in England and Wales. The Environment Agency does not recommend or support this option due to the potential for both Health and Safety Issues and Emissions to arise. No further guidance is provided in this section with regard to this option. To facilitate Producer Audit criteria all waste collections and deliveries should be prearranged and supervised by someone technically aware of the waste types permitted for the transfer or treatment operations on the site. Waste acceptance is divided into two stages - Acceptance procedures prior to delivery. - Acceptance procedure on delivery The following Table sets out the Waste Acceptance, Tracking and Labelling requirements for Clinical Waste only. 3.1 : Stage 1 - Prior to delivery (producer audit option) 1. From the waste disposal enquiry the Licence holder should obtain information in writing relating to: The details of the healthcare waste producer The specific process from which the waste derives – veterinary, primary care, dental, acute, laboratory etc. The quantity of each waste type Compositional audit analysis of the waste (individual constituents of each waste stream and their percentage compositions and chemical/pharmaceutical contaminants) 10 of 48 DRAFT FOR COMMENT The form the waste takes and the types of containers used (colour, form and size of container and sub-containers) Hazards associated with the waste and its components. Date of production of the waste and waste storage and preservation techniques used since production (for example cold storage, or freezing, that may impede treatment.) 2. Unless an audit analysis has already been completed and the Licence holder has sufficient written information to support this, the Licence holder should in every case obtain representative audit analysis of the waste from the premises of production / current holder and compare it with the written description to ensure that it is consistent. The audit data should be less than 18 months old. 3. The type of information that would demonstrate the reliability of the audit includes: A diagram of the producer premises indicating the location, numbers, types and capacities of waste containers in use. A list of the different wards, departments, or functional areas that exist within the premises. A list of those areas that were included within the audit, and the containers within those areas that were examined. A date and description of the audit and the procedures employed. A confirmation of the number of containers of each type audited and a detailed list of the contents and labelling of each. Compositional audit analysis of the waste (individual constituents of each waste stream and their percentage composition and chemical/pharmaceutical contaminants). The waste classification and disposal options for the constituents of each stream. Where relevant, the audit should include examination of the segregation of waste containers placed in on site bulk containers (e.g. 770 litre carts). A summary report indicating the findings for each area in the producer premises and each waste stream produced there. 4. For pure product chemicals, laboratory smalls, or pharmaceutical waste containers, the audit can include reference to product data sheets or an extrapolation of information on product data sheets. 5. Following characterisation of the waste, a technical assessment should be made of its suitability for treatment or storage to ensure compliance with licence conditions. 6. Wastes should not be accepted at the site without a clear method or defined treatment and disposal route being determined in advance. 7. There must be a clear distinction between sales and technical staff roles and responsibilities. If non-technical sales staff are involved in waste disposal enquiries, then a final technical assessment prior to approval should be made. It is this final technical checking that should be used to avoid build-up of accumulations of wastes. 8. All records relating to pre-acceptance should be maintained at the site for crossreference and verification at the waste acceptance stage. These records should be kept for a minimum of 2 years, or 3 years where required by the Hazardous Waste Regulations. 11 of 48 DRAFT FOR COMMENT It is not unusual for the licence holder to also act as the carrier and collect the waste from the producer’s premises. In these instances, this can be the initiation of the second waste acceptance stage. Waste acceptance must ensure that there are 2 levels of acceptance, the first being acceptance checking and the higher level being that of audit. Clinical waste is usually bagged or sealed in UN approved packaging and then placed in larger carts for transportation. The contents of all carts should be visually inspected upon receipt to ensure that the carts do not contain obvious nonconforming wastes (for example waste containers of a type associated with an unsuitable waste, or waste types/containers not identified specifically on the documentation). Where it is not possible to determine visually that non-conforming wastes are absent, procedures must be put in place to unload the cart, with due consideration for both emissions and health and safety. If it is not possible to unload the cart to achieve this, then the waste must be sent for incineration. The following box includes indicative standards for Clinical Waste only. 3.2: Stage 2 - Procedures when waste arrives at the facility (producer audit option) 1. On arrival loads should: Be weighed unless alternative reliable volumetric systems are available Not be accepted into site unless sufficient storage capacity exists and the site is adequately manned Have all documents checked and approved, and any discrepancies resolved before the waste is accepted 2. Where possible confirmatory checks should be undertaken before offloading where safety is not compromised. Visual inspection of the waste within the ‘carts’ must in any event be carried out immediately upon offloading at the site. 3. Every container should be checked to confirm quantities against accompanying paperwork. All containers should be clearly labelled and should be equipped with well fitting lids. 4. At this stage the waste tracking system should begin. A unique reference number should be applied to each container. Each container should also be labelled with the date of arrival on site. 5. The Licence holder should ensure that waste delivered to the site is accompanied by a written description of the waste describing its composition, hazard characteristics and handling precautions, compatibility issues, and information specifying the original waste producer and process. 6. Documentation provided by the driver, written results of acceptance analysis, details of offloading point or off-site transfer location should be added to the tracking system documentation. 7. A record of the inspection regime for each load and justification for the selection of this option should be maintained at the site. 12 of 48 DRAFT FOR COMMENT 8. Should the inspection or analysis indicate that the wastes fail to meet the acceptance criteria then such loads should be stored in a dedicated quarantine area and dealt with appropriately. The maximum storage time for such loads should take account of the potential for odour generation and insect infestation. In all cases the maximum storage time for waste that has failed to meet the acceptance criteria should be five days working days. Written procedures should be in place for dealing with wastes held in quarantine, together with a maximum storage volume. 9. The offloading, sampling point/reception and quarantine areas should have an impermeable surface with self-contained drainage, to prevent any spillage entering the storage systems or escaping off site. All surfaces should be of sufficient type and quality to allow effective disinfection. 10. The Licence holder should have clear and unambiguous criteria for the rejection of wastes, together with a written procedure for tracking and reporting such nonconformance. This should include notification to the customer/waste producer and Regulator. Written/computerised records should form part of the waste tracking system information. The licence holder should also have a clear and unambiguous policy for the subsequent storage and disposal of such rejected wastes. This policy should achieve the following: Identifies the hazards posed by the rejected wastes Labels rejected wastes with all information necessary to allow proper storage and segregation arrangements to be put in place Segregates and stores rejected wastes safely pending removal 11. The waste tracking system should hold all the information generated during preacceptance, acceptance, storage, treatment and/or removal off-site. Records should be made and kept up to date on an ongoing basis to reflect deliveries, on-site treatment and despatches. The tracking system should operate as a waste inventory/stock control system and include as a minimum: Date of arrival on-site Producers details All previous holders A unique reference number Pre-acceptance and acceptance analysis results Package type and size Intended treatment/disposal route Accurate records of the nature and quantity of wastes held on site, including all hazards and identification of primary hazards Where the waste is physically located in relation to a site plan Where the waste is in the designated disposal route Identification of licence holders staff who have taken any decisions re acceptance or rejection of waste streams and decided upon recovery/disposal options 12. All records relating to pre-acceptance should be maintained and kept readily available at the site for cross-reference and verification at the waste acceptance stage. Records should be held for a minimum of two years after the waste has been treated or removed off site. 13. The system adopted should be capable of reporting on all of the following Total quantity of waste present on site at any one time Breakdown of waste quantities being stored pending treatment Indication of where the waste is located on site relative to a site plan Comparison of quantity on site against total permitted 13 of 48 DRAFT FOR COMMENT Comparison of time the waste has been on site against permitted limit 14. Back up copies of computer records should be maintained off-site. 15. Wastes should not be accepted at the site without a clearly defined method of recovery or disposal being determined and sufficient capacity being available. These checks should be performed before the waste acceptance stage is reached. 16. There must be a clear distinction between sales and technical staff roles and responsibilities. If non-technical sales staff are involved in waste enquires then final technical assessment prior to approval should be made. It is this final technical checking that should be used to avoid build up of accumulations of wastes and to ensure that sufficient capacity exists. 14 of 48 DRAFT FOR COMMENT 4 Waste Storage and Disinfection The following guidance on the handling and storage of clinical waste are made in addition to the recognised standard requirements that apply generally to waste facilities and are intended to reduce the potential for Spillage, resulting in the release of aerosols and litter. • Odour. • Infestation by pests. • Interference by trespassers. 4.1 Handling of Waste on Arrival. Good practice is for waste to be transported in rigid leak proof containers. Any waste arriving without such containers, e.g. loose yellow bags, should be unloaded from the delivery vehicle directly into rigid leak proof containers for transport around the site. The integrity of waste packaging should be protected at all times. Waste should not be unloaded from rigid leak proof containers after arrival on site to facilitate onward transport. 4.2 Separation of waste types There should be demarcated storage areas for different waste streams to ensure that the wastes streams are not mixed. In particular the following must be kept separate from other wastes: Bagged clinical waste Waste containing chemicals Anatomical wastes Cytotoxic and cytostatic medicine contaminated waste. Waste medicines. Amalgam Sharps boxes Non-clinical offensive/hygiene wastes Treated outputs waste from the process. A separate, secure and clearly labelled Quarantine Area should be provided for waste that the facility is not authorised to accept for treatment or transfer. 4.3 Storage of Waste All clinical waste should be kept in totally enclosed, clearly labelled and secure areas Sited on an impermeable pavement with sealed or foul drainage system. Sharps boxes may be stored either in 15 of 48 DRAFT FOR COMMENT Leakproof rigid containers the lids of which shall be kept closed when the bin is not being loaded or unloaded, or a designated secure area of a building. Anatomical waste and any wastes with the potential to produce odours should be stored securely in designated refrigerated units within a building. Chemical and pharmaceutical waste should be stored within a designated secure area of a building in manner that is consistent with their chemical properties. (REFERENCE?) Other wastes should be stored in leak proof rigid containers the lids of which shall be kept closed when the container is not being loaded or unloaded. Waste in rigid packaging, e.g. anatomical waste, sharps and pharmaceutical waste, should be stored in an upright and controlled manner to minimise the potential for spillages. Note that certain sharps boxes may not be designed to retain fluids, and may therefore release fluids if stored or handled in an inappropriate manner. Treated waste outputs from the process should be stored in enclosed containers that prevent its escape. The wastes should be identified and handled as difficult wastes warranting specific handling procedures, for example, at a landfill site such procedures may include directing the wastes to the base of the working face for immediate cover. Routine monitoring of waste stored on-site should be carried out by checking and recording storage facilities to ensure that bags or containers are intact and there is no leakage of any fluids. The manner in which waste is store should facilitate this. 4.4: Cleaning of Storage Areas and containers The surfaces of the storage areas should be of sufficient type and quality to allow effective disinfection. Site surfaces and the surfaces of fixed storage containers should be cleaned and disinfected regularly. Once emptied all re-usable mobile rigid containers should be checked to ensure all waste has been removed. These containers should be cleaned and disinfected appropriately after use, and in particular on each occasion prior to their removal from the site, in order to prevent fugitive emissions entering the hospital environment. Wash waters must be contained within an impermeable area to prevent flow of runoff into external areas or to surface water drains. Wash waters must be drained to foul sewer or disposed of appropriately. 4.5: Compaction of clinical waste There should be no compaction of clinical waste. Compaction of non-clinical offensive/hygiene wastes has the potential to produce emissions and is not considered best practice. 16 of 48 DRAFT FOR COMMENT Any compaction procedure for non-clinical offensive/hygiene should take place on an impermeable surface and must both minimise the release of and include the monitoring for the release of; Micro-organisms, Bio-aerosols and liquid discharges. 4.6: Duration of Storage Clinical Waste has the potential to produce odour and to attract vermin or pests if the waste is not processed directly upon arrival at a transfer or treatment site. This depends on a number of factors including the age of the waste, type of waste, ambient conditions, and integrity of packaging As a result no particular storage time should be specified in a licence. It is recommended that that licence conditions are used to control any potential problems arising from the storage of clinical waste e.g. odour and vermin. The site operating procedures should however indicate the maximum duration of storage for each waste type accepted by that facility for treatment or onward transfer. It is recommended that this should be no longer than 14 days for pharmaceutical waste, and 7 days for any other waste stream. Facilitate the treatment of waste in rotation based on identification of its age on arrival and duration of storage on site. Where waste is sent for onward transfer it should be sent directly to the final treatment or incineration facility to reduce the potential for extended residence times, and generation of odour, elsewhere in the waste chain. 4.7: Contingency Technical breakdowns are not uncommon at clinical waste treatment facilities. Where they have occurred this may result in excessive, inappropriate and prolonged storage and potential breaches of a range of licence conditions. Cessation of treatment or waste acceptance at the treatment plant may cause similar problems at the transfer stations that supply the treatment plant. Both treatment and transfer sites should not accept waste without a clearly defined method of recovery or disposal being determined and sufficient capacity being available. These checks should be performed before the waste acceptance stage is reached. ensure that they have formal contingency plans in place to maintain compliance with licence conditions and routine operating procedures. This should include alternative disposal sites for waste, with due consideration of waste rejection procedures under the Hazardous Waste Regulations. The contingency plans should not refer to other sites or companies without documented agreement from those parties to act as the contingency. 17 of 48 DRAFT FOR COMMENT 4.8: Site access and security Certain clinical wastes present particular security concerns. The waste facility should be securely fenced and gated to prevent unauthorised external access. A security co-ordinator should be appointed to ensure that good practice security arrangements are in place at the site. Such measures may include alarms, CCTV and use of security guards as appropriate to the location, scale and type of facility. Facilities handling Group D waste will need to operate a higher degree of security and record keeping to prevent unauthorised tampering with, or theft of pharmaceuticals. 18 of 48 DRAFT FOR COMMENT 5: Treatment Efficacy for Alternative Treatment Processes 5.1 : Introduction The fundamental principle of any Alternative Treatment (Disinfection) is that it renders infectious clinical waste safe i.e. it removes that hazard that makes the waste clinical waste. The efficacy of a particular treatment process is a measure of its ability to render clinical waste safe. There are three stages in the Quality Assurance of treatment efficacy. 1. Process Efficacy Testing – to demonstrate that the intended process, for example the one to which a licence application relates, has previously been proven to be effective elsewhere. 2. Site Commissioning Validation – to demonstrate that each device installed on the site is able to treat the waste that is being subjected to the process, using the parameters and operational procedures employed by that facility. Data from the testing of one item of equipment cannot be used for an identical item of equipment in operation in the same or different premises as consistent construction, performance and operation cannot be guaranteed. Generic testing of a technology, rather than individual items of equipment, may form part of the Process Efficacy Testing but cannot be considered for Site Commissioning Validation. 3. Routine Monitoring – ongoing monitoring of treatment efficacy to support real time parametric records and controls. There are four criteria that must be met to render a clinical waste safe a. for infectious waste – the treatment must demonstrated the ability to reduce the number of organisms present in the waste to a level that no additional precautions are needed to protect workers or the public against infection by the waste; b. for anatomical waste – destroys any human or animal issue, organ or body so that it is no longer generally recognisable. c. for any clinical waste – renders any syringes, needles or any other equipment or item unusable and no longer in their original shape and form (unrecognizable); d. for medicinal waste – destroys the component chemicals. 5.2 : For infectious waste In the USA, the State and Territorial Association on Alternate Treatment Technologies (STAATT) has provided four levels to define the levels of microbial inactivation required for clinical waste treatment. The 1998 STAATT guidance is accepted by the Environment Agency as the basis of the minimum standards for the treatment of infectious waste in England and Wales. These principles are adopted into this guidance with consideration of domestic legislation, current knowledge, and awareness of the forthcoming revision of the STAATT guidance. 19 of 48 DRAFT FOR COMMENT STAATT Level I Description Inactivation of vegetative bacteria, fungi and lipophilic viruses at a 6 log10 reduction or greater. Level II Inactivation of vegetative bacteria, fungi, lipophilic/hydrophilic viruses, parasites and mycobacteria at a 6 log10 reduction or greater. Level III Inactivation of vegetative bacteria, fungi, lipophilic/hydrophilic viruses, parasites and mycobacteria at a 6 log10 reduction or greater; and inactivation of B. stearothermophilus or B. atrophaeus spores at a 4 log10 reduction or greater. Inactivation of vegetative bacteria, fungi, lipophilic/hydrophilic viruses, parasites and mycobacteria and B. stearothermophilus spores at a 6 log10 reduction or greater. Level IV To avoid the need to test treatment plants with a range of micro-organisms it is considered acceptable to use thermally or chemically resistant bacterial spores as pathogen surrogates. These are substantially more resistant to treatment than most vegetative bacteria, fungi, viruses and parasites. There inactivation can normally be assumed to be indicative that other micro-organisms have also been inactivated. Key requirements for Infectious Waste Treatment 1. The test to establish if the numbers or activity of pathogens has been reduced so that no additional precautions are needed to protect workers or the public against infection by the waste is based on the Level III criteria recommended by the State and Territorial Association on Alternative Treatment Technologies (STAATT). 2. The level IV criteria must be met for the treatment of certain biohazardous waste 3. The above level III or IV criteria must be demonstrated for the worst-case scenario challenge loads e.g. a rigid, 2 litre, gel-filled chest drain/suction canister. This principle is implicit in the STAATT guidance. A worst-case challenge load must include waste articles that are likely to be present in the waste treated at the site that inhibit treatment. A challenge load would be expected to include items that provide thermal insulation or prevent chemical penetration e.g. rigid 2 litre gel filled chest drain/suction canisters. Chemical process validation should include a high organic load challenge. 4. Where the waste is macerated or shredded prior to the thermal/chemical treatment process, then the challenge load may be the size-reduced waste. 5. Where the waste is reduced in size as an integral process that occurs simultaneously with the thermal/chemical process then the use of size-reduced waste as the challenge load is not appropriate. 6. Treatment Efficacy shall be validated and tested in accordance with the standards detailed in Appendix A. 7. The device should not process waste in a batch quantity or throughput rate 20 of 48 DRAFT FOR COMMENT greater than that assessed during validation. 8. Mobile plant should be fully validated on commissioning and undergo confirmatory tests before commencing operations on another site. Full validation will be required if the device is to be resident for more than 6 months. 5.3: For anatomical waste Alternative treatments are not considered appropriate for the treatment of anatomical waste. This waste has three particular properties that underpin this The ability of tissue, during or after treatment, to generate odour The longer time taken treat larger pieces of tissue The ethical issues associated with such treatment, particularly with the shredding of such waste. New technologies may enter the market that have the potential to destroy either Anatomical waste, and/or Anatomical wastes contaminated with pharmaceuticals (including cytotoxic and cytostatic medicines). Incineration reduces anatomical waste to ash. The successful application of alternative treatments to this waste stream must achieve the equivalent result. Alkaline hydrolysis for example has the potential to digest the flesh from a carcass leaving only a bone shadow. Validation of such technologies would have to demonstrate the ability to destroy the tissue of worst case scenario anatomical wastes. Consideration would also have to be given to the presence of pharmaceutical and infectious contaminants in such waste, as discussed in section 2.8.5. 5.4: For any Clinical Waste Clinical waste that is subjected to alternative treatment may contain items or disposable equipment that should be shredded/macerated to render it unrecognisable and prevent its reuse. This would also act to prevent any patient information being determined from labels within the waste. All such treatments should be capable of reducing the waste, by maceration, shredding or other means, to a particle size of less than or equal to 50mm with no particle exceeding 80mm in any dimension. Any plant that macerates/shreds clinical waste that has not already been rendered safe should also be designed and built specifically to ensure microbiological aerosol containment. This should include operation under negative pressure, with air drawn away from the hopper entrance and passed through HEPA filters. Hoppers should have doors on the opening to retain aerosols. Microbiology laboratory autoclaves, which treat a more limited and less recognisable range off microbiologically contaminated waste from that laboratory, on the premises of the laboratory are not required to meet this criteria as long as either 21 of 48 DRAFT FOR COMMENT The partially treated waste is subsequently shredded before final disposal to landfill, or The partially treated waste is classified as offensive waste (e.g. 18 01 04 or 18 02 03), and disposed of with additional precautions at landfill. Treatment plants for clinical waste, other than microbiology laboratory autoclaves, are expected to have in place procedures to achieve the particle sizes specified above. 5.5: For Medicinal Waste Treatment for Pharmaceutical and pharmaceutically contaminated Wastes (e.g. sharps) requires that all pharmaceutically active substances present in the waste, both hazardous and non-hazardous, should be destroyed during treatment. At present, high temperature incineration is the only recognised method of achieving this. It is possible that future alternative technologies, for example alkaline hydrolysis, may demonstrate treatment efficacy in this Area. In general this would require laboratory ‘process efficacy’ trials to demonstrate that process parameters are theoretically able to treat a very broad range of representative and worst case scenario pharmaceutical molecules before site commissioning validation protocols for device based trials could be considered. The temperatures required to destroy a number pharmaceuticals are known to significant beyond those currently employed non-incineration thermal processes. Chemical treatments should also be viewed with caution as they may result in; No reaction with the pharmaceutical, (i.e. the pharmaceutical is untreated) A reaction that destroys the pharmaceutical producing a non-hazardous reaction product. A reaction that results in the production of a hazardous reaction product. No distinction is made in this section between the treatment of cytotoxic and cytostatic medicines and the treatment of other medicines classified as nonhazardous in the List of Wastes. Both groups are typically designed to interact with biological systems at very dilute concentrations and may possess a range of hazardous properties. ‘Dilution’ with other waste or ‘small quantities’ do not equate to efficacy of treatment. 5.6 Parametric monitoring and controls Parametric monitoring of a clinical waste treatment process should provide real-time data acquisition for assessing efficacy. The data acquired from the parametric monitoring device(s) must be correlated with the requirements for rendered safe during Site Commissioning Validation. The parametric monitoring and controls should be:- 22 of 48 DRAFT FOR COMMENT based on automated rather than manual recording of critical parameters. Include tamper-proof controls or automatic factory-set controllers such that critical parameters cannot be altered during routine operations and cycle formats other than those that have been validated cannot be used. integrated with the treatment unit to automatically shut-down or no longer accept or expel waste if treatment conditions are not maintained at specified performance levels. derived from appropriately and periodically calibrated monitoring devices capable of accurately measuring key process parameters, for example time, temperature, pressure, humidity, chemical volume and active agent concentration. 5.7: Process Efficacy Testing This is information from a similar device located elsewhere in the UK or overseas submitted in support of a licence application to demonstrate that the proposed technology has previously demonstrated efficacy of treatment elsewhere. Data from sources outside the United Kingdom should be treated with a degree of caution. In many cases the regulatory regime, guidance, licence holder procedures and healthcare waste segregation practices will differ to extent that such data is only an indicator of the potential performance of a device. No criteria are set for this step, other than data relating to process efficacy testing should be submitted with the licence application. The purpose of collecting this information is to confirm that the technology is robust enough for a licence to be issued, and to assist the licence holder and regulator in identifying any known issues with the technology that might affect the next step in the assessment, Site Commissioning Validation. Licences for processes treating anatomical or medicinally contaminated waste should not be issued without detailed submissions on efficacy of the process being submitted and technically assessed by specialists. 5.8: Site Commissioning Validation All clinical waste treatment devices must be validated once they have been installed on site and prior to commencing treatment operations. This validation demonstrates that the device works to the required level of treatment when employed by that licence holder for the waste streams they accept. This validation is valid for a maximum of 48 months, and should then be repeated to ensure that changes in the waste stream composition and ‘wear and tear’ have not impaired performance. This is required for newly installed equipment and in cases where existing plant is shut down because it failed to demonstrate routine efficacy. Validation must demonstrate that the plant can meet the requirements of rendered safe, for example for infectious waste - STAATT level III (or where applicable IV) using methodology consistent with the guidance presented in this appendix A. 23 of 48 DRAFT FOR COMMENT Waste treatment operations should not commence until the Environment Agency has technically considered the Validation report and confirmed its agreement in writing that the criteria have been met.. Procedures for anatomical waste, pharmaceuticals and pharmaceutically contaminated waste are not provided. However the general principles of Appendix A bases on statistical reproducibility and worst case scenario should be adhered to rigorously. Procedures for ‘unusable and unrecognisable’ – this can be conducted by physical measurement of particle size. NOTE : Investigations by the Overseas bodies, the National Health Service, Health Protection Agency, and Environment Agency have identified significant issues with previous validation methodology, and its application to many technologies. Environment Agency officers are recommended to seek specialist advice from NOPTS Technical Advisors before agreeing that a report meets the standards indicated. 5.9 : Routine Monitoring All clinical waste treatment devices should be monitored routinely throughout their operational life to ensure that the requirements for rendered safe are met and that this performance is maintained. The frequency of the monitoring should consider the Parameters of rendered safe being monitored The economics of the monitoring which may relate to the throughput of the device. The ease of monitoring, which may be device dependent. The presence of robust parametric controls and automated recording that reduces the need for frequent monitoring. The frequency should never however be less than annual and where any concerns or uncertainties remain (for example due to absence of supporting monitoring data in process efficacy submissions) should be more frequent. Appendix B provides the minimum requirements for infectious waste, with consideration of the above criteria, for those units that have robust parametric controls and automated recording in line with 2.8.6. 24 of 48 DRAFT FOR COMMENT 6: Emissions & Monitoring 6.1: Introduction Potential emissions from clinical waste sites can be divided into the following categories Pathogenic micro-organisms Chemicals and pharmaceuticals Nuisance – litter, offensive litter, dust, odour and noise. Pests and vermin. 6.2: Pathogenic Micro-organisms Clinical waste can be reasonably expected to contain pathogens. Although the total number of micro-organisms (both pathogenic and non-pathogenic) may be less than that found in domestic refuse, it is likely that a wider range of pathogens will be present and that some of these will have the ability to either cause more severe symptoms or possess some form of resistance to anti-microbial therapies that could complicate treatment. These emissions must therefore be minimised and regulated. Examples of where emissions may arise include : Emissions to air resulting from breached packaging during handling procedures. Emissions to air from the process, particularly during shredding of untreated waste. Transmission beyond the site boundaries by infection or contamination of staff or visitors leading to spread in the community Transmission beyond the site boundaries by infection or contamination of, or the removal of infected material by, pests or vermin. Transmission beyond the site boundaries by the return of contaminated waste containers to healthcare premises. Transmission beyond the site boundaries by reloading bagged waste, other than in wheeled carts, on vehicles for onward transfer in a manner that causes breaches in the packaging. The three main concerns are Release of bioaerosols Spillages of bodily fluids including blood, faeces, vomit, sputum, etc. Needlestick injuries Aerosol emissions from point sources such as steam pressure relief valves should be prevented by the appropriate use of high efficiency particulate air (HEPA) filters. Indicative requirements for the control of point source emissions to air 1. HEPA filters should be effectively maintained to ensure a minimum particle removal efficiency of 99.97% for all particles of 0.3m diameter. 2. Procedures should be in place to allow for the safe removal and disposal of HEPA filters. 25 of 48 DRAFT FOR COMMENT Plant that macerates/shreds clinical waste has the potential to generate fugitive emissions of pathogens to air. Any plant that macerates/shreds clinical waste that has not already been rendered safe should also be designed and built specifically to ensure microbiological aerosol containment. This should include operation under negative pressure, with air drawn away from the hopper entrance and passed through HEPA filters. Hoppers should have doors on the opening to retain aerosols. Releases to foul sewer are less likely to present a risk, however monitoring of such releases may enable identification of failures in plant integrity. 6.3: Chemicals and Pharmaceuticals A wide range of both pharmaceuticals and chemicals is used in healthcare. These may possess a wide range of chemical risk phrases and therefore hazardous properties. Known issues include: Incompatible reactions, for example resulting in spontaneous combustion. Leakage from containers, for example sharps boxes, that are not designed to be leak proof. Volatile chemicals, for example formaldehyde, released during thermal treatments. Discharges of organic chemicals to foul sewer, particularly from condensers of steam based processes. Consideration should also be given to the discharge of disinfectants used in chemical treatment and site cleaning processes. 6.4 : Nuisance – litter, offensive litter, dust, odour and noise. Clinical waste, like many wastes, has the potential to generate litter. Specific concerns relate to: Contaminated items within the waste being released with secondary consequences. Items within the waste that are recognisable and may cause significant offence, for example items of human tissue. Information relating to a patient, for example labelled sample containers or patient record. The potential for litter or dust can be reduced by transporting packaged clinical waste both into and out of the site in large wheeled carts, and similarly treated clinical waste in enclosed containers. The potential to generate odour should be considered when determining the length of time clinical waste is stored, the type of container used for storage and the temperature at which waste is stored. On site procedures should minimise the time between the production and final disposal of the waste. Prolonged storage and movement from one transfer station to another are bad practice. The mechanical components of the treatment have the potential to generate noise. 26 of 48 DRAFT FOR COMMENT With consideration of the above points General Environment Agency guidance on the regulation of noise, odour, dust and particulates should be followed, at the time of publication this is Environment Agency Noise Guidance, Internal Guidance for the Regulation of Noise at Waste Management facilities, version 3.0, July 2002. Environment Agency Odour Guidance, Internal Guidance for the Regulation of Odour at Waste Management facilities, version 3.0, July 2002. Monitoring of Particulate Matter in Ambient Air around waste Facilities – Technical Guidance Document (Monitoring) – M17, version 3.0, February 2003 6.5 : Pests and vermin The organic constituents of clinical waste have the potential to attract insects, birds, rodents, and other scavenging mammals in the same fashion as domestic refuse. Live maggots are now in general use within the National Health Service for treatment of wounds. The waste itself therefore has the potential to produce one or more generations of flies within waste containers if conditions permit. Additional concerns relate to The infection or contamination of pests or vermin by materials in the waste The removal of particularly offensive items of waste by pests or vermin. It must be remembered that clinical waste streams may include waste from veterinary practices that presents an infection risk to other animals, or in some cases (Zoonotic diseased) to man as well. Waste from human healthcare that presents an infection risk to people, or in some cases to animals as well. Animals may serve as a vector or reservoir of both human and animal disease. Site Operating Procedures should include regular monitoring for pests and vermin, and remedial actions to be taken if found. 6.6. Spilllages 6.6.1: Introduction The primary objective is to prevent a spillage occurring through use of Robust waste acceptance to identify wastes on arrival, and Correct storage of those wastes, and Maintaining the integrity of waste packaging. Design and construction of appropriate site surfaces and drainage Where a spillage has occurred it is important to consider the nature of the material and the appropriate method to manage it : Biological contamination should be treated with disinfectants. Chemical or pharmaceutical contamination should be cleaned up with appropriate chemical containment procedures. This is likely to depend on the nature of the chemical. Whether the material is liquid or solid is also likely to be relevant. Records should be kept of the time, place, nature, cause and remedial action taken with regard to each spillage. 27 of 48 DRAFT FOR COMMENT Further information is available from Environment Agency Pollution Prevention Guidelines 6.6.2 : Spillage infrastructure and equipment Waste facilities should have basic spillage equipment that is easily accessible in case of accidental spillage. Supplies of spillage kits with suitable absorbent material must be kept on site and all staff should be trained in their use. All site licence holders should be aware of where the equipment is located and it should consist of: A suitable disinfectant Suitable absorbent materials. Scoops. Additional protective clothing to that already used by site licence holders. Disposable cloths and paper towels. Waste disposal bags and rigid containers to collect the spillage. 6.6.3 : Biological contamination and disinfectants Disinfectants may have differing spectrums of activity against micro-organisms, may possess hazardous properties, and may be affected by organic matter in the waste. It is important that broad spectrum disinfectants are used in appropriate quantities and concentrations, and that the potential for chemical releases or reactions is minimised. Should a spillage occur from a clinical waste container, the liquid spill and/or the area contaminated by solid waste should be treated with an effective chlorine based disinfectant. Sodium dichloroisocyanurate (NaDCC) granules are recommended for the clean-up of spillages as other made-up solutions lose activity with time and require regular replacement. Disinfectants containing 10,000ppm (1%) available chlorine are recommended for spillages. Suitable inert, absorbent materials may be used to deal with liquid spillages after disinfection. The nature of disinfectants for use in both routine cleaning and spillage clean-up should be discussed with the trade effluent control staff of the sewage undertaking. The impermeable surfaced areas of the facility should be regularly cleaned and disinfected and it is recommended that, generally, this should be done on a monthly basis. This frequency should be increased in areas prone to frequent leakage. 6.6.4: Chemical spillages Spillages of process chemicals (for example, from chemical treatment processes) or disinfectants should be cleaned up in accordance with recommendations on the Manufacturers Safety Data Sheets (MSDS) and the COSHH assessments made by the licence holder. Spillages of fuel, oil, lube oil etc should be absorbed using appropriate spillage kits. Once the spill has been absorbed and the adsorbent removed, surfaces should then be steam cleaned or pressure washed. 28 of 48 DRAFT FOR COMMENT 6.6.5: Disposal of spillage materials Once the clean-up of a spillage has been completed, the material should be immediately placed in an appropriate container (e.g. clinical waste bag, sharps bin or chemical spill bin). Once this has been done, the wastes should be sent for treatment or disposal at an appropriately licensed waste facility. Depending on the nature of the spill or the content of the chemical used to clean the spillage, the package may have to be consigned as Hazardous Waste 6.6.6: Site Surrender criteria It is recommended that where an application is made to surrender a waste management licence at a clinical waste facility, other than landfills, the following should be carried out: remaining wastes must be removed. process equipment must be thoroughly cleaned and disinfected or removed. storage containers must be thoroughly cleaned and disinfected and removed. The site must be decontaminated by thoroughly cleaning and disinfecting hard surfaces, drains and interceptors. The site licence holder must provide details of spillages or pollution incidents that occurred throughout the life of the site and how they were dealt with. 29 of 48 DRAFT FOR COMMENT Appendix A : Site Commissioning Validation for Infectious Waste Treatment A1: Introduction This section contains the detailed minimum requirements for demonstration of disinfection efficacy for Site Commissioning Validation of clinical waste treatment device. These are presented as General requirements - that apply to all plants Supplementary Specific procedures – that apply to different technologies. A1: General Requirements Validation Report 1. A validation report should contain the following elements: i) A microbial efficacy analysis, that demonstrates that the choice of test organism, the method of introduction to the plant, the choice of organism carrier, and the analytical method are adequate to demonstrate STAATT level III criteria for the worst case scenario challenge load. ii) Evidence that effective parametric controls, and procedures for real-time monitoring and assessment of outputs, are in place with respect to any waste treated. iii) Evidence that the parametric control data relates to microbial efficacy so that waste can therefore be considered to be treated satisfactorily on the basis of parametric controls alone. iv) An environmental monitoring assessment of the site that addresses process emissions, including emissions from the macerator/shredder. v) Where procedures differ from (i) evidence that operational efficacy monitoring regimes are effective. 2. For newly installed treatment plant operations must not commence until the licence holder has submitted the report to the Regulator for assessment and has received from the Regulator written confirmation that the validation report has been agreed. Commissioning of the Plant 3. The procedures and criteria that must be satisfied to demonstrate that treatment can achieve the microbial inactivation aspect of ‘rendered safe’ is specified in the following sections depending on the type of plant that is being commissioned: A2: Procedure for Microbial Validation for pre-maceration technologies where spore strip integrity can be guaranteed (for examples Augers). A3: Procedure for Microbial Validation for pre-maceration or integral maceration technologies where spore strip integrity cannot be guaranteed (for example Autoclave with integral macerator). A4: Procedure for Microbial Validation for technologies that lack premaceration or integral maceration 4. Both the field and laboratory aspects of this procedure should be carried out by a suitably qualified microbiologist utilising a suitably accredited laboratory. Test Organism 5. For thermal and chemical processes the tests should be performed using either 30 of 48 DRAFT FOR COMMENT Bacillus atrophaeus (subtilis) OR Geobacillus stearothermophilus. 6. The Licence holder should demonstrate that the choice of spore species, strain and certification is the most appropriate for the treatment method employed. 7. A single batch number of spore strips / spore suspension should be employed during commissioning. 8. Each batch of spores will have a certified D-value. The D-value is the time taken, in minutes, for a 1 log10 reduction, in the number of spores exposed to specified conditions. Not all batches of spores will have the same D-value. This D-value may vary by up to 100% for commercially available spores of the same type, and variance beyond this range is available on request. The choice of spore strip may therefore increase or reduce the number of spores recovered by a factor of 10. This can therefore alter the reported reduction by up to 2 log10. 9. To maintain consistency of treatment standard, the STAATT level III criteria should be demonstrated using spores where the certified D-value is 2 minutes - at 121C wet heat (Geobacillus stearothermophilus) - at 160C dry heat (Bacillus atrophaeus) 10. Where the certified D-value of a batch of spores is < 2 minutes, or determined at parameters other than those identified above, they are not suitable for use. This applies to both routine monitoring and validation. A2: Procedure for microbial validation for pre-maceration technologies where spore strip integrity can be guaranteed (for examples Augers). 1. This applies only to those technologies that have pre-maceration and where it is possible for the test materials to be inserted easily into the macerated waste prior to it entering the microbial treatment process. Spore Strip Containment 2. Spore strip challenges should be carried out on the technology using spore carriers where the integrity of the container can be guaranteed. 3. Spore carriers should be designed to mimic normal conditions in the waste being treated as much as possible, and the type chosen will be dependant on both the technology and the waste treated. Examples that may be suitable include net bags, tennis balls with holes in them, socks, plastic containers with holes in or alloy containers with holes in. 4. Where spore strips are placed in metal containers they must always be wrapped in a layer of cotton wool, or equivalent, to prevent direct conduction of heat from the metal. 5. Where technologies have integral mixing it is not appropriate to attach the spore carriers to the mixing arms because the waste is not fixed to the mixing arms i.e. 31 of 48 DRAFT FOR COMMENT the spore carriers should be loose in the waste. The licence holder may attach up to 50% of the spore carriers to fixed positions, however the results for these must be assessed separately, and both fixed and loose spore carriers must pass the criteria provided. If the licence holder can demonstrate statistically that there is no significant difference between the two data sets, then routine operational monitoring can use fixed carriers. 6. Similar criteria apply to the use of test ports into which spore strips can be placed. In general these are not representative of the treatment to which the waste is subjected and should not be used. Outline Test Procedure 7. If the technology processes the waste in batches, the tests should be carried out over a minimum of 5 separate treatment cycles for each cycle format. For example where the device is operated at two different temperatures or times, each must be validated separately using 5 cycles. 8. For continuous technologies, the tests should be done in five distinct collections for each cycle format, with the tests for each previous collection being retrieved from the treated waste before the next set of tests is introduced to the treatment plant. 9. The minimum number of spore strips required is set out in table A2.1 10. Strips should be spread out in the load as much as is practicable. For technologies with integral mixing this may be accomplished inside the machine. For static technologies the strips should be spread out throughout the length of the chamber, and should be placed as near the centre of the load as possible. 11. A minimum of 2 untreated control strips should be held outside the autoclave during each cycle/batch and processed along with the tests afterwards to provide an estimate of the numbers of spores retrievable from each strip. 12. The use of surrogate waste is not appropriate. Clinical waste of the type(s) to be treated by the technology under normal conditions should be used for all tests. 13. All waste ‘treated’ during testing should be either: re-treated by another technology to ensure it is rendered safe, or it should be quarantined until the validation report is agreed by the Regulators (The storage requirements in this document should be complied with). 14. For thermal processes, the microbial data should be supported by the parallel use of thermal indicator strips or multi-point thermal data loggers to record temperatures throughout the waste load. These strips should be chosen to support the parametric controls and measure both temperature achieved, and duration of exposure to that temperature. Laboratory Methods 15. These will be partly determined by the test organism, the method specified by the 32 of 48 DRAFT FOR COMMENT spore supplier, and reference to other guidance on microbiological methods. 16. 100% of each test sample must be analysed. This is not required for control samples. Analysis must be quantitative. Validation Criteria 17. This requires quantitative enumeration of spore strips with a certified population of >1 x 106 spores per strip. Qualitative analysis and/or the use of lower numbers are not appropriate for plant commissioning. 18. For the Control Run(s) the following are required: The mean number (XC) of spores recovered from the control strips should be calculated in cfu. The log10 of (XC) should be determined. 19. For the Test Runs the following require determining: The mean (XT) number of spores recovered The standard deviation () of spores recovered The log10 of (XT) The Upper 95% (Lu) confidence interval of (XT) (this will be XT+ 1.96) The log10 of the Upper 95% confidence interval (logLu) of XT 20. The following criteria represent the minimum standard that must be achieved: (log (XC)-4) logLu log (XC) must be 5 For thermal processes all thermal indicator strips should indicate that the required temperature time parameters have been achieved. 21. These criteria must include the proviso that ALL test strips, or spore samples, recovered from the plant must be considered valid. This includes those where contamination has occurred. Significant contamination will therefore require the exercise to be repeated. 22. Where these criteria are passed then it is 97.5% probable that any clinical waste will be treated to the minimum required standard. Table A2.1: Minimum number of spore strips required for microbial validation of Alternative Treatment Plants with Pre-maceration technologies where spore strip integrity can be guaranteed. Single Load Capacity Minimum Number Minimum Minimum Number (Kg) of spore strips per Number of spore of control strips Continuous cycle or collection. strips (for each cycle throughput (Kg per (for each cycle format) Hour) format) 0-10 kg 3 15 10 33 of 48 DRAFT FOR COMMENT 11-50 kg 51-250 kg 251-500kg 501-750kg >750 kg. 6 9 12 15 18 30 45 60 75 90 10 10 10 10 10 A3 : Procedure for microbial validation for pre-maceration or integral maceration technologies where spore strip integrity cannot be guaranteed (for example Autoclave with integral macerator). 1. This applies to those technologies with integral maceration, or pre-maceration that cannot easily be by-passed. It does not apply to those technologies with integral mixing or fragmentiser arms that do not meet the definition of maceration. (e.g. Hydroclaves and Rotaclaves.) 2. The ‘fixing’ of spore strips, in such a way as to bypass the integral maceration process, is not considered to be appropriate. This may overestimate the efficacy of the treatment process and is not representative of the processes to which the waste is subjected. 3. Where the integrity of the containers cannot be guaranteed, it is necessary to use spore suspensions. 4. The spore suspension is mixed with the waste before treatment, and sufficient of the treated waste is collected after treatment to allow a numerical count of the number of surviving spores to be made. For each run the spore suspension should be placed in at least 6 discrete portions in representative challenge waste items, for example inside syringe bodies in sharps boxes, inside suction canisters or chest drains etc. 5. This procedure requires: One control run, where waste is passed through the device with the thermal/chemical treatment inactivated. This provides an estimate of spore recovery from the waste. Then three test runs should be performed for each treatment cycle format. These data are compared with the control run to provide an estimate of treatment efficacy. 6. The control run is required because of the ‘natural’ loss of spores with this procedure due to absorption or trapping of spores in the materials in the waste, the dilution factor, and the inadequacies of the retrieval and concentration process. 7. For chemical processes, it is essential that the device be thoroughly cleaned to remove residual traces of disinfectant prior to conducting control runs. 8. For health and safety reasons it may be appropriate to use for the control run either: A batch of treated clinical waste, or A prepared batch of clinical waste composed of uncontaminated items 34 of 48 DRAFT FOR COMMENT Required testing parameters 9. For thermal processes the waste should be seeded with sufficient of a > 1 x 1010ml–1 spore suspension to achieve at least 1 x 106 spores per gram throughout the load. (this equates to at least 0.1 ml per kg of waste). Geobacillus stearothermophilus is strongly recommended in order to reduce the interference from other microbes. 10. Sub-samples of the treated waste should be collected from throughout the load and analysed separately - 0-50 kg per cycle/hour – test using a minimum of 3 sub-samples per cycle/batch - 50-500 kg per cycle/hour – test using a minimum of 4 sub-samples per cycle/batch - >500 kg per cycle/hour – test using a minimum of 5 sub-samples per cycle/batch 11. Each sub-sample should equate to at least 0.1% of the waste load, with the minimum sub-sample size set at 50g for smaller units. The sub-sample size should equate to at least 1 x 107spores. 12. Analysis is complex, the following being an indication of a typical procedure rather than an approved and accredited method. Expert advice should be sought before conducting such analysis. Samples should be appropriately preserved until received by the laboratory and subjected to testing. The testing must commence within an appropriate time scale. The entire sub-sample is mixed with excess sterile physiological saline for at least 15 minutes on an orbital shaker. (Note that neutralising buffer may be required for chemical treatments) The liquid is decanted through a sterile coarse fabric filter to remove solid waste. The liquid is centrifuged at 3000g for 20 minutes to deposit the spores. The deposit is resuspended in 10 ml of brain heart infusion broth (BHI). (additional washing of the deposit in saline/buffer may be necessary prior to this step) Serial dilutions are made in BHI from 1:10 to 1: 1,000,000 These should be analysed in triplicate in thick pour plates Plates are incubated in a moist chamber at 60°C for up to 7 days. 13. For thermal processes the microbial data should be supported by the parallel use of thermal indicator strips or multi-point thermal data loggers to record temperatures through out the waste load wherever possible. Validation Criteria 14. This requires quantitative enumeration of the sub-samples relative to the control run. Qualitative analysis or the use of less than 1 x 106 spores per gram is not appropriate. 15. For the Control Run(s) the following are required The mean number (XC) of spores recovered from the control samples should be calculated in cfu. The log10 of (XC) should be determined. 16. For the Test Runs the following require determining 35 of 48 DRAFT FOR COMMENT The mean (XT) number of spores recovered The standard deviation () of spores recovered The log10 of (XT) The Upper 95% (Lu) confidence interval of (XT) (this will be XT+ 1.96) The log10 of the Upper 95% confidence interval (logLu) of XT 17. The following criteria represent the minimum standard that must be achieved: (log (XC)-4) logLu log (XC) must be 5 For thermal processes all thermal indicator strips should indicate that the required temperature time parameters have been achieved. 18. These criteria must include the proviso that ALL test strips, or spore samples, recovered from the plant must be considered valid. This includes those where contamination has occurred. Significant contamination will therefore require the exercise to be repeated. 19. Where these criteria are passed then it is >97.5% probable that any clinical waste will be treated to the minimum standard. A4 : Procedure for microbial validation for technologies that lack premaceration or integral maceration 1. These technologies may have severe limitations and may lack the technical ability to treat a worst-case scenario challenge load of clinical waste. Environment Agency officers should always seek specialist advice before issuing an authorisation, or other approval, for such technologies. 2. Where there is no physical action to enable sealed waste containers, and sealed voids in the waste to be punctured, then the treatment is unlikely to penetrate the waste fully. 3. In general, specialist challenge load testing using methodology consistent with that developed by the Health Protection Agency would be required to confirm efficacy. This is beyond the scope of this document. 4. Static autoclaves, including those with vacuum cycles, are particularly affected by this issue and the waste will require either Some form of physical pre-treatment (e.g. maceration), and/or an extended cycle duration to enable effective treatment to take place. 5. As an indicator of methodology requirements, spore strips should be placed in each of the following: Robust rigid 2 litre suction canister/chest drain containers made of thermostable plastic, of variable types containing 1-1.5 litres of fluid and thermally stable gel. The licence holder should demonstrate that the type(s) chosen represent the worst case challenge load., and Any other challenging items identified by audit where the penetration of the steam/chemical may be inhibited (for example lengths of tubing, inside 36 of 48 DRAFT FOR COMMENT syringe bodies in sealed sharps boxes etc). 6. Testing should cover 18 - 36 suction canisters and chest drains (in 3-6 test runs per plant), including approximately 6 of each type/brand, each containing two biological indicator strips and two thermal indicator strips. 7. These containers should be placed in rigid containers and/or yellow bags of the type to be taken by the plant, and to reflect normal operations, and mixed with a typical waste load. 8. The validation criteria from section A3, 17-22 should be applied. Commercial Off-site Treatment Facilities 9. Commercial treatment facilities receiving waste from producers located elsewhere should always be tested in line with paragraph 5 above, using the worst case scenario challenge load in use by any healthcare waste producer. On site Treatment Facilities 10. Treatment facilities located on, and serving only, the premises of production of the waste have three options, either Option 1; Test for the general worst case scenario challenge load, as indicated in paragraph 9 above for Off-site treatment facilities. This option is the Environment Agency’s recommended option, OR Option 2; Test as indicated in paragraph 5 above, using the worst case scenario item generated by that premises of production. If this option is chosen the licence holder must demonstrate that they have sufficient knowledge, control and monitoring of waste production and on-site purchasing such that the producer of the waste would not be able to introduce a more challenging item into the waste without their knowledge. This might for example be more applicable for devices intended to operate within specialist units. OR Option 3; Segregation of difficult to treat items. In that case the device must be tested on the basis of the remaining worst case scenario challenge load identified from waste composition analysis. If this option is chosen the licence holder must demonstrate that they have sufficient knowledge, control and audit monitoring of waste segregation such that the producer of the waste would not be able to introduce a more challenging item into the waste without their knowledge. Whichever option is chosen the device must routinely operate on the worst case scenario load cycle selected and validated. 37 of 48 DRAFT FOR COMMENT Appendix B : Routine Efficacy Monitoring All clinical waste treatment devices should be monitored routinely throughout their operational life to ensure that microbial inactivation is occurring and that performance is maintained. The following is considered to be the minimum requirements for such monitoring. B1: For those plants for which the use of spore strips is appropriate 1. The minimum frequency of monitoring is specified in Table B1 2. For thermal processes, thermal indicator strips or multipoint data loggers should always be used in parallel where possible. 3. Either qualitative or quantitative enumeration of spore strips with a certified population may be used. 4. Controls and certificates from the test batch should also accompany each set of samples. 5. The quantitative criteria for success are as follows 95 % of the individual spores strips, with a population of >1 x 106, in the first 6 months of operation , and each calendar year, should demonstrate 4 log10 inactivation or higher For thermal processes thermal indicator strips should accompany each spore strip and indicate that the minimum time and temperatures have been achieved for 99% of spore strips. The number and type of spore/thermal indicator strips used, and the frequency of spore testing throughout the calendar year is uniform. For each calendar year a summary report should be prepared that indicates the results obtained and any failures. The data should be referenced to the validation report to demonstrate that predicted treatment efficacy, rather than minimum standards, are being achieved. 90% of spore results should demonstrate a level of inactivation the 95% confidence level of treatment determined during validation. 6. The qualitative criteria for success are as follows 95 % of the individual spores strips, with a population of >1 x 104, in the first 6 months of operation, and each calendar year, should demonstrate no growth. For thermal processes thermal indicator strips should accompany each spore strip and indicate that the minimum time and temperatures have been achieved for 99% of spore strips. The number and type of spore/thermal indicator strips used, and the frequency of spore testing throughout the calendar year is uniform. For each calendar year a summary report should be prepared that indicates the results obtained and any failures. Where >5% (or 1, whichever is greater) of spore strips exhibit growth in any calendar year quantitative testing should be used in future instead of qualitative testing. 38 of 48 DRAFT FOR COMMENT 7. These criteria must include the requirement for all test strips to be valid. The percentage allowance has been provided to allow for both potential contamination and the uncertainty of microbial data. 8. If at any time during the calendar year it becomes clear that these criteria cannot be met for that year, the Regulator should be informed immediately, and the plant should cease operations until such time as the cause can be identified and remedied to the satisfaction of the Regulators. In order to recommence operations additional validation is required (see Appendix A.) 9. In any other circumstances, where the licence holder becomes aware that one or more batches of waste may not have been treated to the required standard, the Licence holder is expected to take appropriate action. Table B1 Routine Monitoring of Microbial Inactivation where the use of spore strips is appropriate Continuous Test Test Minimum Number of Hourly frequency frequency Number spore Control throughput or (first 6 (operational, strips or sub- strips batch cycle months of after the first samples load.(kg) operation) 6 months) 0-50kg Monthly quarterly 5 1 51-500 kg Fortnightly Bi-monthly 5 1 501-1000kg Weekly monthly 5 1 B2 : For those plants for which suspension testing is required. 1. The minimum frequency of monitoring is specified in Table B2 2. For thermal processes, thermal indicator strips or multipoint data loggers should be used in parallel where possible. 3. Quantitative enumeration of spore suspensions with a certified population is required. 4. A single control run is required. 5. The number of test runs and sub-samples per test run is indicated in Table B2. 6. In other respects: the procedures in section B1 the quantitative criteria for success from B1 Note : The Environment Agency accepts that Site Commissioning Validation may use both spore suspensions, and spore strips fixed within the main body of the waste, solely for the purpose to demonstrating that spore strips can be used for routine monitoring. This requires a demonstration that statistically the same results are achieved by either method. This does not extend to the placement of spore strips in any other location, for example in sampling ports. 39 of 48 DRAFT FOR COMMENT Table B2 : Routine Monitoring of Microbial Inactivation Where Suspension testing is required Continuous Hourly Test frequency Test frequency Number of Number throughput or (first 6 months (operational, samples test runs batch cycle of operation) after the first 6 subload.(kg) months) samples per test run 0-250kg 6 monthly Annually 3 1 251-750 kg 6 monthly Annually 6 2 751+kg Quarterly 6 monthly 6 3 40 of 48 of DRAFT FOR COMMENT Appendix C : Emissions Monitoring & Benchmarks It should be recognised that emissions from technically sound clinical waste treatment plants, operated under good practice with appropriate containment, and treating appropriate waste should be low. Where waste acceptance or pre-acceptance procedures are poor, and/or containment is inadequate or unproven, this assumption cannot be made. The onus is therefore on the licence holder to demonstrate that emissions from the plant are controlled during both site commissioning and more importantly during routine operation. Potential emissions include Microbes, particularly in the form of bioaerosols Chemicals, particularly volatile organic carbons. Formaldehyde is of particular concern. Pharmaceuticals, as a source of complex chemical emissions. Microwaves, from containment failures of microwave technologies. Examples of most of the above have previously been identified as significant issues on clinical waste treatment sites. The purpose of this section is to ensure that monitoring is in place to identify failures in the design, integrity, containment or operation of the site or process. Reference should be made to Agency Guidance M17 Monitoring of particulate matter in ambient air around waste facilities. 1: Microbial Emissions Monitoring. Microbial monitoring is required, as there is the potential for aerosols containing pathogenic organisms to be released during the operation of alternative waste treatment plants. Potential sources include: During maceration of untreated clinical waste The release of exhaust gases. During maceration of treated clinical waste. Failures in plant integrity One of the problems associated with such monitoring is the variation of types and number of microbes within the load. Determining which ones to monitor for, and the quantitative relevance of any detected, is difficult to ascertain. Several may also arise from other sources and therefore be unrelated to plant emissions. The following procedure using tracer spore suspensions is recommended. However it should be noted that monitoring of other indicators may be undertaken as an alternative to tracer spore suspension where demonstrated by the licence holder to be appropriate. The procedures for such monitoring should be agreed with the Regulator prior to implementation. 41 of 48 DRAFT FOR COMMENT Procedure for Microbial Emissions Monitoring using tracer spore suspension. For technologies that shred or macerate the waste prior to treatment 1. A dry or liquid suspension of bacillus spores should be prepared and dispensed (in a laboratory environment) in a number of sealed, small volume plastic containers. These should be dispersed throughout the waste load and processed. For other technologies 2. Dry or liquid suspensions of bacillus spores should be prepared and dispensed (in a laboratory environment) both loosely in waste inside containers (bags, boxes etc), and inside worst case challenge load containers (suction canisters/chest drains). These should be dispersed throughout the waste load and processed. 3. Spore strips should never be used for bioaerosol emissions monitoring. 4. The quantity of spores should equate to a minimum of 1 x 106 spores per gram of total waste load. 5. All devices should be tested, during commissioning validation, during the first six months of operation and periodically thereafter as indicated in Table A6-2.10.4 6. Process emission monitoring should continue throughout the operational life of the plant. Frequency of Testing 7. The minimum frequency of monitoring is specified in Table C1 Sampling Methodology 8. The sampling should consist of air monitoring and surface monitoring 9. The number of samples and location of sampling points will depend on the nature of the process and size of the device. Recommended sample locations are specified under the respective headings of Air Monitoring, Surface Monitoring and Wastewater Discharge Monitoring. 10. The sampling programme should be designed to take sufficient samples to enable the results to be quantitatively related to the input dose. 11. Samples should be taken: - prior to the processing of the seeded waste (controls), - at intervals during the processing of the seeded waste (the intervals should relate to process stages and timing of potential emissions), and - periodically thereafter for at least 2 hours after the cycle is complete 12. The aim of the monitoring programme is to produce a quantitative ‘estimate’ of the total number of tracer organisms emitted from the device relative to the input dose by each route. Air Monitoring 13. Air monitoring should be conducted around identified point source emissions from the process, as well as at the site boundaries, and at any other relevant locations within the site – for example open vehicle access doors to building within which the plant is located. 42 of 48 DRAFT FOR COMMENT 14. Key examples of emission sources:Point Source Emissions The main point source emission to air is from the venting of exhaust gases. Exhaust gases should always be treated (e.g. filtered through a HEPA filter). Monitoring is required to demonstrate that the treatment of the gases has been effective and should take place at each emission point Sources of fugitive Emissions include: i) Maceration of untreated clinical waste. This is potentially the most significant source of pathogenic bioaerosols. Monitoring should demonstrate that containment measures in place are effective. ii) Maceration of treated clinical waste may also result in the generation of bioaerosols as treatment is required to reduce the number of microorganisms rather than eliminate them. This monitoring should demonstrate if additional containment measures are required. iii) Maintenance or access ports. Monitoring is necessary to ensure that these do not compromise the integrity of the plant, that they are effectively sealed during operation, and that emissions are not released. 15. It is recommended that active (centrifugal/vacuum) impaction onto agar using Anderson or slit samplers, or equivalent, is used to sample for bioaerosols. Data submissions should contain information indicating the recovery efficiency of the method used. 16. Monitoring should be conducted throughout the emissions monitoring exercise, and with individual sample times to coincide with steps in the process where emissions may occur (for example the passage of seeded waste through a shredder). Surface Monitoring 17. To support the air monitoring outlined above, it is recommended that settle plates are employed in large numbers to form a grid-like pattern around the device/site. 18. The exposure time for each plate, and replacement frequency during testing may need to consider contaminants and total microbial load. 19. The use of a regular exposure time, a series of plates at each sampling point, and a grid placement should enable an estimation/calculation of the total number of organisms that have settled during the monitoring period for Each grid square, and For the whole site. This can be compared to the input dose to provide a quantitative release estimate for the process. Wastewater Discharge Monitoring 20. Where the process produces a wastewater this should also be monitoring at intervals during the testing. For chemical processes, the potential need for neutralisation of disinfectant should be considered. 21. The purpose of this additional monitoring is to ensure that both the Treatment process is operating effectively; and 43 of 48 DRAFT FOR COMMENT The wastewater arises post treatment 22. Wastewater should be sampled prior to entering the drainage system, and as near to the point of origin as possible. Table C1 - Process Bioaerosol Emissions Monitoring When a suspension of Bacillus Spores has been used First 6 Subsequently Minimum months (if proven and Number of agreed) Sampling points For devices which shred/macerate untreated waste For other devices Quarterly Annually See text Minimum Number of samples per sampling point See text 6-monthly Bi-annually See text See text 2 : Chemical Emissions Monitoring Waste acceptance and pre-acceptance procedures are intended to prevent waste containing chemicals entering the treatment process. There are two primary consequences where these procedures are insufficient : volatile chemicals are released to atmosphere/water . This is a particular concern with thermal processes. Incompatible reactions occur. This is a potential concern with chemical treatment processes. During validation, a written assessment of the Volatile Organic Compounds (VOCs) emitted from the process shall be submitted to the Agency. The written assessment shall include: a scale drawing showing location of the emission points monitored; sampling of the emission and comparison against the benchmark values listed in Section 3.11 of the Sector Guidance Note IPPC S5.06, dated December 2004, to assess their significance; proposal of any necessary modelling of the emission; and details of how any emissions are to be prevented during the operation of the facility. particular attention should be paid to VOC’s that are associated with the healthcare waste stream (for example Formaldehyde) VOC monitoring should take place during commissioning, after the first 6 months of operation, and annually thereafter. Thermal process (e.g. autoclaves) that employ water condensers should monitor liquid discharges for VOC’s. For chemical processes consideration should be given to possible emissions from the chemical agent used, and any products known to arise from its reaction with the waste. 44 of 48 DRAFT FOR COMMENT 3 Pharmaceuticals emissions monitoring Waste acceptance and pre-acceptance procedures are intended to prevent waste containing pharmaceuticals, including fully discharged medicinally contaminated syringes, entering the treatment process. Monitoring of emissions from such substances would not normally be required where the licence holder can demonstrate that waste acceptance and pre-acceptance procedures are sufficient to exclude such waste. 4: Microwaves emissions monitoring Licence holders of microwave facilities should be aware that failures in containment might result in leakage of non-ionising radiation and have operational procedures to check for such leakage at regular intervals. 5 : Criteria for Success The licence holder should monitor and react to changes, trends and patterns in emissions. For example a gradually increasing trend around the site may require improvements in site procedures generally, whilst a sudden increase of emissions around the shredder may indicate a failure of a specific containment feature. Emission Benchmarks 6: Emission benchmarks Table C2 details emission benchmarks for point source emissions from the clinical waste site. These best practice benchmarks are not mandatory release limits. Table C2 : Emission Benchmarks Emission Air – sample points <10m from the treatment plant. Water Measure Cfu Unit Bacillus 1000 Per cubic metre1 spores Bacillus (300)2 Per litre1 spores Note 1 : These Units relate to the overall monitoring period so the cfu benchmark applies to Each individual sample of air taken, with a calculation made to report the result per cubic metre. For each individual settle plate (this is not an average)– a calculation made to adjust for surface area of a settle plate and exposure time (for example if settle plates are deployed for only 15 minutes of every hour then the result must be multiplied by 4). Each individual sample of water taken, with a calculation made to report the result per litre. Note 2: These benchmarks are indicative only, and will be reviewed periodically. Table C3 provides additional guidance with respect to fugitive emissions from the site, these can be used to support the emission benchmarks in Table C 45 of 48 DRAFT Table C3 : Emission of spiked organisms Emission Measure Cfu Unit Air – sample points>10m from the Bacillus 300 Per cubic metre1 treatment plant spores Surface – sample point < 10m Bacillus (20000)2 Per square metre from the treatment plant spores per hour 1 Surface – sample points > 10 m Bacillus (5000)2 Per square metre from the treatment plant. spores per hour 1 Note 1 : These Units relate to the overall monitoring period so the cfu benchmark applies to Each individual sample of air taken, with a calculation made to report the result per cubic metre. For each individual settle plate (this is not an average)– a calculation made to adjust for surface area of a settle plate and exposure time (for example if settle plates are deployed for only 15 minutes of every hour then the result must be multiplied by 4). Each individual sample of water taken, with a calculation made to report the result per litre. Note 2: These benchmarks are indicative only, and will be reviewed periodically. 46 of 48 DRAFT Appendix D : Modern Regulation Introduction The treatment of clinical waste requires either a Waste Management License or a Pollution Prevention and Control Permit. The Environment Agency recognises that there are two specific circumstances where is may not be proportionate, or in the public interest, to require a license or permit. Laboratory autoclaves, and Small clinical waste treatment devices on the premises of production The Environment Agency must balance this with significant concerns over treatment efficacy, producer segregation, and the potential impact of this proposal on the marketplace The following is proposed : Proposed Position The treatment of infectious waste on the premises of production of that waste in accordance with the criteria identified in Appendix D of the Environment Agency Technical guidance on Clinical Waste Management facilities are met. Criteria 1 : Laboratory Autoclaves - The device (or devices) is situated on the laboratory premises of production of the waste the licence holder would be the waste producer if a licence were required. treats only infectious waste generated by that laboratory in containment level 1-3 facilities. The total quantity of infectious waste treated on the site by laboratory autoclaves is less than 1 tonne per day. is validated in accordance with HTM 2010 or equivalent for the worst case scenario challenge loaded generated by that laboratory to achieve a minimum temperature of 121’C for a minimum time of 15 minutes within the core of that challenge load. Equivalent to STAATT level IV using Geobacillus stearothermophilus. 2 : Other Clinical Waste Treatment - The device (or devices) is situated on the premises of production of the waste the licence holder would be the waste producer if a licence were required. treats only infectious waste generated by that producer of the types indicated as suitable in section 2.2, excluding those indicated as unsuitable in section 2.3. is supported by the waste acceptance audit procedures identified in section 3. The total capacity of the infectious waste treatment devices of any type on the site, excluding laboratory autoclaves, is less than 200kg tonne per day. Has demonstrated efficacy in accordance with Section 5 and methodology consistent with Appendix A, and the site commissioning validation report has been agreed in writing with the Environment Agency. This report agreement is 47 of 48 DRAFT valid for a period of 48 months from the date of agreement. Operations should not commence until the report has been agreed. The site commissioning must be repeated and resubmitted for agreement for a further 48 months. Incorporates a shredding/maceration (or other equivalent destruction step) to the standard specified in section 5.4 (recommended), or is disposed of as offensive/hygiene waste with appropriate procedures at the receiving landfill. The two options are provided separately in consideration of the different nature of the waste they treat, established operational practices, the differential treatment standards applied, and significant concerns with both waste segregation and device validation with regard to the option 2. 1 References APPENDIX 6 Sector Guidance Note IPPC S5.06 – Supplementary PPC for Clinical Wastes Safe Disposal of Clinical Waste, 1999, Health Services Advisory Committee, ISBN 07176 2492 7. Safe Management of Healthcare Waste: A Public Consultation, Gateway No 5471, Department of Health. www.dh.gov.uk. Technical Guidance WM2, Hazardous Waste, Interpretation of the Definition and Classification of Hazardous Waste, Environment Agency, ISBN 1 84432 4540. Clinical Waste Disposal/Treatment Technologies (alternatives to incineration), Health Technical Memorandum HTM 2075, NHS Estates, The Stationery Office, ISBN 0-11322159-2. CONTROL OF AEROSOL (BIOLOGICAL AND NONBIOLOGICAL) AND CHEMICAL EXPOSURES AND SAFETY HAZARDS IN MEDICAL WASTE TREATMENT FACILITIES FINAL REPORT Contract No. 200-95-2960 RTI Project No. 93U-6449 Prepared For National Institute for Occupational Safety and Health, November 1997 Non-incineration Medical Waste Treatment technologies, Healthcare without harm, August 2001, http://www.noharm.org/details.cfm?type=document&id=919 Non-incineration Medical Waste Treatment technologies in Europe, without harm, June 2004, http://www.noharm.org/europe/medicalwaste/nonincineration Healthcare Safe management of Wastes from Healthcare Activities. World health Organisation, 1999, http://www.healthcarewaste.org/en/documents.html?id=1 48 of 48