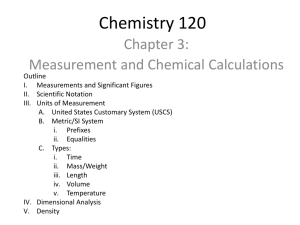
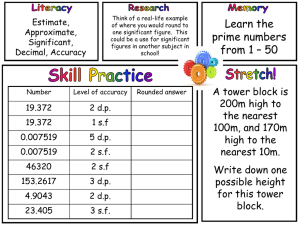
Significant Figures Calculations Worksheet
advertisement

Significant Figures Calculations Solve the following mathematical problems such that the answers have the correct number of significant figures: 1) 334.54 grams + 198 grams = 2) 34.1 grams / 1.1 mL = 3) 2.11 x 103 joules / 34 seconds = 4) 0.0010 meters – 0.11 meters = 5) 349 cm + 1.10 cm + 100 cm = 6) 450 meters / 114 seconds = 7) 298.01 kilograms + 34.112 kilograms = 8) 84 m/s x 31.221 s = This work is licensed under a Creative Commons Attribution - NonCommercial 4.0 International License. More chemistry tutorials and practice can be found at www.chemfiesta.com. Significant Figures Calculations Answers Solve the following mathematical problems such that the answers have the correct number of significant figures: A handy tip: Remember that addition/subtraction has different rules when doing significant figure calculations than multiplication/division. 1) 334.54 grams + 198 grams = 533 grams. It is rounded from 532.42 grams because “198 grams” is only precise to the nearest gram. 2) 34.1 grams / 1.1 mL = 31 g/mL (it's not rounded) 3) 2.11 x 103 joules / 34 seconds = 62 J/s (rounded from 62.0588 J/s because 34 seconds has only two significant figures) 4) 0.0010 meters – 0.11 meters = -0.11 m (rounded from -0.109 m because 0.11 meters is precise to the nearest 0.01 meters, forcing us to round to the same precision.) 5) 349 cm + 1.10 cm + 100 cm = 500 cm (rounded from 450.1 cm because 100 cm is precise only to the nearest hundred cm) 6) 450 meters / 114 seconds = 3.9 m/s (rounded from 3.9474 m/s because 450 meters has only two significant figures) 7) 298.01 kilograms + 34.112 kilograms = 332.12 kilograms (rounded from 332.122 kilograms because 298.01 kilograms is only precise to the nearest 0.01 kg). 8) 84 m/s x 31.221 s = 2600 m (rounded from 2622.564 m because 84 m/s has only two significant figures). This work is licensed under a Creative Commons Attribution - NonCommercial 4.0 International License. More chemistry tutorials and practice can be found at www.chemfiesta.com.