Supplement 1
advertisement

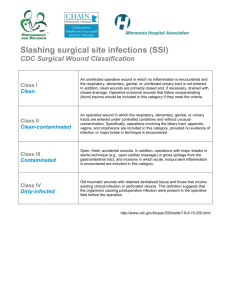
Supplement 1 A. Wound edge protection device (CWEP) used in the intervention arm. B. Control intervention with coverage of the wound edges with sterile surgical towels. Supplement 2 Superficial Incisional SSI 1. Infection occurs within 30 days after the operation AND 2. Infection involves only skin or subcutaneous tissue of the incision AND 3. At least one of the following: A. Purulent drainage, with or without laboratory confirmation, from the superficial incision. B. Organisms isolated from an aseptically obtained culture of fluid or tissue from the superficial incision. C. At least one of the following signs or symptoms of infection: pain or tenderness, localized swelling, redness, or heat and superficial incision is deliberately opened by surgeon, unless incision is culture-negative. D. Diagnosis of superficial incisional SSI by the surgeon or attending physician. Deep Incisional SSI 1. Infection occurs within 30 days after the operation AND 2. Infection involves deep soft tissues (e.g., fascial and muscle layers) of the incision AND 3. At least one of the following: A. Purulent drainage from the deep incision but not from the organ/space component of the surgical site. B. A deep incision spontaneously dehisces or is deliberately opened by a surgeon when the patient has at least one of the following signs or symptoms: fever (> 38ºC), localized pain, or tenderness, unless site is culture-negative. C. An abscess or other evidence of infection involving the deep incision is found on direct examination, during reoperation, or by histopathologic or radiologic examination. D. Diagnosis of a deep incisional SSI by a surgeon or attending physician. Notes: 1. Report infection that involves both superficial and deep incision sites as deep incisional SSI. 2. Report an organ/space SSI that drains through the incision as a deep incisional SSI. Organ/Space SSI 1. Infection occurs within 30 after the operation AND 2. Infection involves any part of the anatomy (e.g., organs or spaces), other than the incision, which was opened or manipulated during an operation AND 3. At least one of the following: A. Purulent drainage from a drain that is placed through a stab wound‡ into the organ/space. B. Organisms isolated from an aseptically obtained culture of fluid or tissue in the organ/space. C. An abscess or other evidence of infection involving the organ/space that is found on direct examination, during reoperation, or by histopathologic or radiologic examination. D. Diagnosis of an organ/space SSI by a surgeon or attending physician. CDC definition of surgical site infections (SSI) (Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999;27(2):97–132). ‡ If the area around a stab wound becomes infected, it is not an SSI. It is considered a skin or soft tissue infection, depending on its depth. Supplement 3 Monitoring of trial data was performed by an independent institution experienced in the monitoring of surgical trials (Münchner Studienzentrum, MSZ). Monitoring was carried out in accordance with ICH-GCP guidelines and standard operating procedures of the MSZ to ensure patients´ safety, integrity of clinical data and adherence to study protocol. Extend of the monitoring was prespecified in a monitoring manual before initiation of the trial. Amongst others informed consent forms, inclusion and exclusion criteria, the evaluation of the primary endpoint and blinding was verified by on-site monitoring in all patients on the basis of source documents. All trial sites were activated with an initiation visit by the monitor who handed out and explained the investigator site file, discussed relevant issues and trained trial personnel in study-specific interventions. Supplement 4 Safety analysis of adverse and serious adverse events other than SSIs in the control group (towels; n=294 patients) and intervention group (CWEP; n=300 patients). A total of 11 patients died within 45 days of surgery (four in the control group and seven in the interventional group). None of the deaths was associated with the device. Furthermore, no device related adverse events were reported. Analysis of adverse events gives adverse event counts (not patient numbers). One patient may experience more than one adverse event. Numbers in brackets indicate percentage of all adverse events. There were 456 adverse events (AEs) other than SSIs in 267 patients reported in the intention to treat population (289 AEs in 158 patients in the control group; 167 AEs in 109 patients in the CWEP group). The most frequent AEs were fever, anemia, increase in C-reactive protein, urinary tract infections and leukocytosis. Of the 456 AEs 94 were serious adverse events (SAEs). The most frequent SAEs were respiratory failure, bowel obstruction, acute renal failure and pneumonia. AE=adverse event; SAE=serious adverse event; SSI=surgical site infection; UTI=urinary tract infection; CRP=C-reactive protein; GI=gastrointestinal. Control (towels) Intervention (CWEP) 289 167 57 (19.7%) 37 (22.2%) Fever 34 (11.76%) 18 (10.78%) Anemia 23 (7.96%) 19 (11.38%) CRP-increase 17 (5.88%) 11 (6.59%) UTI 15 (5.19%) 13 (7.78%) Leukocytosis 15 (5.19%) 9 (5.39%) Ileus 12 (4.15%) 6 (3.59%) Pneumonia 8 (2.77%) 4 (2.4%) Tachycardia 9 (3.11%) 2 (1.2%) Hypokalemia 6 (2.08%) 5 (2.99%) Pleural effusion 7 (2.42%) 4 (2.4%) Renal Failure 6 (2.08%) 2 (1.2%) Diarrhea 2 (0.69%) 4 (2.4%) Ascites 5 (1.73%) 1 (0.6%) Urinary retention 1 (0.35%) 4 (2.4%) Nausea & vomiting 3 (1.04%) 2 (1.2%) Atrial fibrillation 3 (1.04%) 1 (0.6%) Art. Hypertension 3 (1.04%) 1 (0.6%) Pancreatic fistula 1 (0.35%) 2 (1.2%) Delayed gastric emptying 2 (0.69%) 1 (0.6%) Port infection 2 (0.69%) 0 Respiratory failure 4 3 Bowel obstruction 2 4 Acute renal failure 2 2 Pneumonia 2 2 Dehiscence of fascia 3 1 Postoperative hemorrhage 4 0 Pulmonary embolism 1 2 Adverse events (including SAEs) Serious adverse events (% of AEs) Most common AEs (% of AEs) Serious adverse events Pneumothorax 2 1 GI hemorrhage 2 1 Sepsis 3 0 Heart failure 2 0 Cerebral insult 2 0 Bowel ischemia 2 0 Multiorgan failure 1 1 Pleural effusion 1 1 Allergic reaction 1 (Amoxicillin) 0 Ascites 1 0 Aspiration 1 0 Asystole 0 1 Myocardial infarction 1 0 Pericardial effusion 1 0 Pleural empyema 0 1 Urinary retention 1 0 Hyperglycemia 0 1 Hepatorenal syndrome 1 0 Hypovolemic shock 0 1 Delayed gastric emptying 0 1 Pancreatic fistula 0 1 Thrombosis of port catheter 0 1 Bile leak 0 1 Others 18 11 Supplement 5 Nyström et al. reported data from 140 elective colorectal patients and found no benefit of use of a ring drape (10% infection rate in the intervention arm, 9% in the control arm). Although length of follow-up was adequate, sample size was limited. It was unclear whether the device was in place during the entire operation or only upon opening of the bowel. Furthermore, the definition of wound infections applied in this trial differs from current classifications (‘pus emptying spontaneously or upon incision’). In addition, the wound infection rates of merely 9% reported in patients undergoing colorectal surgery could not be reproduced in other prospective trials with blinded follow-up, which unanimously report rates of over 20% for colorectal surgeries 1–3. In line with our results, Reid et al. in 2010 reported a significant benefit of CWEPs in 130 colorectal cases at four Australian centers applying the CDC definition of SSIs and an adequate length of follow-up of at least 30 days (SSI rate 22.7% in the control arm, 3.4% in the intervention arm, p=0.004) 2. Recently, a multicenter RCT at 21 centers across the UK failed to report a benefit of CWEPs in 760 patients undergoing open abdominal surgery (SSI rate 25.4% in the control group and 24.7% in the device group) 4. The reason behind the variance between these results and our results are uncertain as both trials have used comparable interventions, the same definition of SSIs and had adequate follow-up. However, although both trials haven been designed and conducted with high methodological quality and aimed to minimize bias, some noteworthy differences exist. First of all, we included slightly different patient populations and procedures as would have been expected for trials with broad inclusion criteria and high external validity. Furthermore, the incidence of SSI in the UK trial was unexpectedly high, not only in the entire study population, but also in the subgroup of clean operations (SSI rate 33.3% in the interventional arm and 24.1% in the control am). These results are in contrast to data from previous RCTs and observational data which have reported results in line with our findings 1,2,5–7 . Also, the trial by Pinkney et al. was conducted in the UK while our trial took place in Germany, which might account for a number of unreported preoperative, operative and postoperative differences in management, handling or trial conduct. Furthermore, BaFO included elective surgeries only, while less than 50% of cases in the UK trial were elective. As a consequence approximately 20% of surgeries were classified contaminated and dirty in the UK trial. Finally, frequency of follow-up visits was different between both trials. We performed six follow-up visits are prespecified time points, all of which were conducted by blinded clinical review assessors, while Pinkney and colleagues performed two visits by blinded clinicians (between postoperative day 57 and 30-33) and one patient reported assessment between postoperative day 7-30. References 1. Bennett-Guerrero, E. et al. Gentamicin–Collagen Sponge for Infection Prophylaxis in Colorectal Surgery. N Engl J Med 363, 1038–1049 (2010). 2. Reid, K., Pockney, P., Draganic, B. & Smith, S. R. Barrier wound protection decreases surgical site infection in open elective colorectal surgery: a randomized clinical trial. Dis. Colon Rectum 53, 1374–1380 (2010). 3. Howard, D. P. J., Datta, G., Cunnick, G., Gatzen, C. & Huang, A. Surgical site infection rate is lower in laparoscopic than open colorectal surgery. Colorectal Dis 12, 423–427 (2010). 4. Pinkney, T. D. et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ 347, f4305 (2013). 5. Darouiche, R. O. et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N. Engl. J. Med 362, 18–26 (2010). 6. Seiler, C. M. et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541). Ann. Surg 249, 576–582 (2009). 7. Mangram, A. J., Horan, T. C., Pearson, M. L., Silver, L. C. & Jarvis, W. R. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27, 97–132; quiz 133–134; discussion 96 (1999).