CENG 241 Heat and Mass Transfer
advertisement
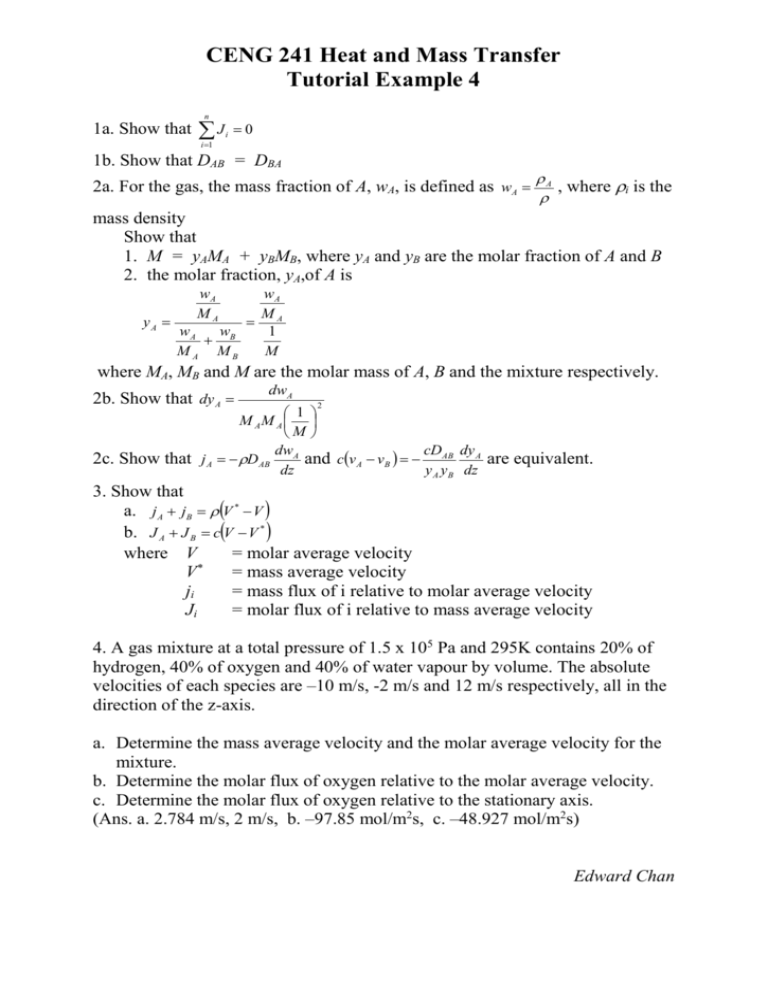
CENG 241 Heat and Mass Transfer Tutorial Example 4 n 1a. Show that J i 0 i 1 1b. Show that DAB = DBA 2a. For the gas, the mass fraction of A, wA, is defined as wA A , where i is the mass density Show that 1. M = yAMA + yBMB, where yA and yB are the molar fraction of A and B 2. the molar fraction, yA,of A is yA wA MA wA w B MA MB wA M A 1 M where MA, MB and M are the molar mass of A, B and the mixture respectively. 2b. Show that dy A dwA 2 1 M AM A M dw cD dy 2c. Show that j A DAB A and cv A vB AB A are equivalent. dz y A y B dz 3. Show that a. j A jB V * V b. J A J B cV V * where V = molar average velocity * V = mass average velocity ji = mass flux of i relative to molar average velocity Ji = molar flux of i relative to mass average velocity 4. A gas mixture at a total pressure of 1.5 x 105 Pa and 295K contains 20% of hydrogen, 40% of oxygen and 40% of water vapour by volume. The absolute velocities of each species are –10 m/s, -2 m/s and 12 m/s respectively, all in the direction of the z-axis. a. Determine the mass average velocity and the molar average velocity for the mixture. b. Determine the molar flux of oxygen relative to the molar average velocity. c. Determine the molar flux of oxygen relative to the stationary axis. (Ans. a. 2.784 m/s, 2 m/s, b. –97.85 mol/m2s, c. –48.927 mol/m2s) Edward Chan CENG 241 Heat and Mass Transfer Solution for Tutorial 4 1a. Ji = ci (vi – V) J i ci vi ciV J i ci vi V ci , as the V is independent of ci c v c c J c v c v 0 J i ci vi i i i i i 1b. i i i i dc A dc and J B DBA B dz dz dc dc c c A cB A B dz dz J A D AB Using Part 1a., JA + JB = 0 D AB DAB dc A DBA dz dc A DBA dz dcB 0 dz dc A 0 DAB DBA dz 2a.1 Recalling the Ideal Gas Law W RT RT M W M PV W RT PAV n A RT y A PAV A RT y A M A WA MA PV W RT PBV nB RT y B PBV B RT y B M B WB MB PV PV nRT PV WA WB W or A B M = yAMA + yBMB AV 2a.2 wA MA nA MA , where V is the total volume wA wB n A nB AV BV MA MB MA MB wA wB 1 M , as wA wB 1 wA wB wA wB wA wB MA MB MA MB MA MB wA M yA A 1 M yA 2b. wA w dw w dw dw B A A A B M MB MA MA MA MB dy A A 2 wA wB M M B A wA + wB = 1 dwA + dwB = 0 dwA = - dwB dy A 2c. 1 wA dwA wA dwA 1 M A M B M A A vA V dwA 1 M A M B M A 2 dwA dz j A DAB * 2 2 1 dy A D AB M A M B , where V* is mass average velocity M dz 2 1 dy A A wA wB v A wAv A wAvB DAB M A M B , wA wB 1 M dz 1 dy A wA wB v A vB DAB M A M B , wA A M dz 2 cv A vB cDAB M AM B wA wB 2 1 dy A M dz 2 M AM B 1 dy A cv A vB cDAB M A y A M B y B M dz M M 2 cDAB 1 dy A cv A vB y A y B M dz 3a. jA + jB = A(vA - V) + B(vB - V) = AvA + BvB - V(A + B) v BvB ( A B ) A A V A B = (V* - V) 3b. JA + JB = cA(vA - V*) + cB(vB - V*) = cAvA + cBvB - V*(cA + cB) c v c V c A cB A A B B V * c A cB = c(V - V*) 4a. In this case, the Ideal Gas Law is modified to PV yi ni RT mi RT , Mi since they have the same pressure but occupy with different portion, yi. PVyi M i vi * RT V mi PVyi M i RT mi vi yM v yM i i i i i V* 0.2 2 10 0.4 32 2 0.4 18 12 2.784 m/s 0.2 2 0.4 32 0.4 18 PVyi vi RT V ni PVyi RT ni vi yv yv y i i i i i V = 0.2 (-10) + 0.4 (-2) + 0.4 (12) = 2 m/s b. Molar flux of oxygen relative to the molar average velocity = cO2 (vO2 - V) PV yO 2 1.5 105 0.4 vO 2 V 2 2 97.85mol/m 2s RT 8.314 295 c. Molar flux of oxygen relative to the stationary axis = cO2 vO2 PV yO 2 1.5 105 0.4 2 48.927mol/m 2s vO 2 RT 8.314 295 Edward Chan










