Name of discharging hospital: Discharge Instruction Sheet for P
advertisement

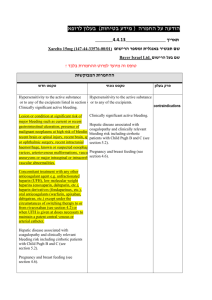
Name of discharging hospital: Discharge Instruction Sheet for Pharmacists Patient’s name Dear This patient has been prescribed the oral anticoagulant Xarelto® (rivaroxaban, Bayer HealthCare Pharmaceuticals) for the treatment of DVT/PE [delete as appropriate] and the prevention of recurrent DVT or PE. This letter provides guidance to support the appropriate use of Xarelto in this patient. Before filling the prescription, please consider the following information about Xarelto: Contraindications to Xarelto include: – Hypersensitivity – Clinically significant active bleeding – Lesion or condition at significant risk of major bleeding – Concomitant treatment with any other anticoagulant agent except under circumstances of switching – Hepatic disease associated with coagulopathy and clinically relevant bleeding risk including cirrhotic patients with Child–Pugh B and C – Pregnancy and breast feeding Xarelto is not recommended in patients who are concomitantly receiving systemic treatment with medications that increase Xarelto plasma concentrations and, therefore, increase the risk of bleeding. This includes (but is not limited to) azole-antimycotics and HIV protease inhibitors, which are both potent inhibitors of cytochrome P450 3A4 and P-glycoprotein Medications that decrease Xarelto plasma concentrations such as phenytoin, carbamazepine, phenobarbital and St John´s wort, which are strong inducers of cytochrome P450 3A4 should be used with caution in patients taking Xarelto Xarelto should be used with caution in patients taking concomitant medications that may affect haemostasis, including ASA or NSAIDs. These patients should be referred to a physician to assess their risk of bleeding Xarelto should be used with caution in conditions with increased risk of haemorrhage and is not recommended in patients with other risk factors for bleeding, including active ulcerative gastrointestinal disease Xarelto is not recommended in patients with PE who are haemodynamically unstable or who may receive thrombolysis or pulmonary embolectomy Xarelto is not recommended for use in patients below 18 years of age 1 This patient was seen by Dr (discharging physician) Contact details Date of discharge Patient information Recommended duration of treatment Additional notes Medication plan for VTE treatment Date and time of first Xarelto dose Other medications Xarelto dosing guide for the treatment of DVT and PE and prevention of recurrent DVT and PE in adults, including those with renal impairment 1 Bayer Pharma AG. Xarelto® (rivaroxaban) Summary of Product Characteristics. November 2012 *Use with caution when concomitantly receiving other medicinal products which increase rivaroxaban plasma concentrations BID = twice daily, OD = once daily Note: The duration of therapy should be individualised after careful assessment of the treatment benefit against the risk of bleeding events 2 Please explain to the patient the importance of taking the anticoagulant as prescribed. The patient should be made aware of signs and symptoms of bleeding and advised to alert a healthcare provider if they experience unexplained dizziness or weakness, swelling or discomfort, sudden severe headache, bruising, bleeding from any site (e.g. nasal, gingival or rectal), heavy menstrual flow or vaginal bleeding, pink or brown urine, pink or black stools, coughing up blood, or vomiting blood or material that looks like coffee grounds. A return to the emergency department may be necessary if the patient experiences significant bleeding from any site, and any other unusual symptoms should be discussed with their general practitioner. The patient should be advised about the importance of notifying any healthcare professional (medical or dental) they visit that they are taking Xarelto. For further information, please refer to the Xarelto Prescriber Aid: https://prescribe.xarelto.com/scripts/index.php Alternatively, the Xarelto (rivaroxaban) Summary of Product Characteristics (approved by the European Commission), is available at: http://www.xarelto.com/html/downloads/Xarelto-Prescribing_Information-Nov-2012.pdf 3