Supplementary Table 2 - Word file (53 KB )
advertisement

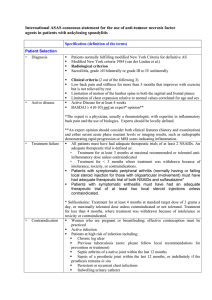
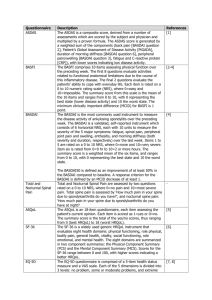
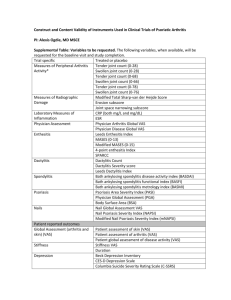
Supplementary Table 2 | Disease activity measures for pediatric and adult SpA* ACR Pediatric Core Set Physician global assessment of disease activity (VAS) Parent/patient global assessment of overall well-being (VAS) Functional ability (CHAQ) Number of joints with active arthritis Number of joints with limited range of motion ESR Improvement defined as at least 30% improvement in 3 of any 6 variables with no more than 30% worsening in any of the remaining variables Wallace criteria for inactive disease and clinical remission of JIA Inactive disease: No joints with active arthritis No fever, rash, serositis, splenomegaly, or generalized lymphadenopathy attributable to JIA No active uveitis Normal ESR or CRP (if both are tested, both must be normal) Physician’s global assessment of disease activity indicates no disease activity Clinical remission: Clinical remission on medication: criteria for inactive disease must be met for a minimum of 6 continuous months while the patient is on medication in order for the patient to be considered to be in a state of clinical remission on medication Clinical remission off medication: criteria for inactive disease must be met for a minimum of 12 continuous months while off all anti-arthritis and anti-uveitis medications in order for the patient to be considered to be in a state of clinical remission off medication BASDAI How would you describe the overall level of fatigue/tiredness you have experienced? How would you describe the overall level of AS neck, back or hip pain you have had? How would you describe the overall level of pain/swelling in joints other than neck, back or hips you have had? How would you describe the overall level of discomfort you have had from any areas tender to touch or pressure? How would you describe the overall level of morning stiffness you have had from the time you wake up? How long does your morning stiffness last from the time you wake up? ASAS core set for disease controlling antirheumatic treatments (DC-ART) Function BASFI Pain VAS (last week/spine/at night due to AS) VAS (last week/spine/due to AS) Spinal mobility Chest expansion Modified Schober Occiput to wall Cervical rotation Lateral spinal flexion or BASMI Patient global VAS Peripheral joints and enthuses Number of swollen joints (44-joint count) Validated enthesitis scores (that is, MASES) X-ray spine Lateral lumbar spine and lateral cervical spine Stiffness VAS (duration of morning stiffness/spine/last week) Inflammatory markers CRP or ESR Fatigue Fatigue question from BASDAI ASDAS Total back pain (BASDAI question 2) Patient global assessment of disease activity Peripheral pain/swelling (BASDAI question 3) Duration of morning stiffness (BASDAI question 6) CRP or ESR ASAS 5/6 improvement criteria Six domains: Patient global (VAS) Pain (VAS) Function (BASFI) Inflammation (mean of BASDAI question 5 and 6) CRP Spinal mobility (lateral spinal flexion) Improvement of > 20% in at least 5 domains *Summarized in ASAS handbook.S7 Abbreviations: AS, ankylosing spondylitis; ASAS, Assessment of SpondyloArthritis international Society; ASDAS, ankylosing spondylitis disease activity score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; CHAQ, childhood health assessment questionnaire; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; JIA, juvenile idiopathic arthritis; MASES, Maastricht Ankylosing Spondylitis Enthesitis Score; SpA, spondyloarthritis; VIS, visual analogue scale. S7. Sieper, J. et al. The Assessment of SpondyloArthritis international Society (ASAS) handbook: a guide to assess spondyloarthritis. Ann. Rheum. Dis. 68 (Suppl. 2), ii1–ii44 (2009). 1