Supplementary Table 2 - Word file (53 KB )
advertisement
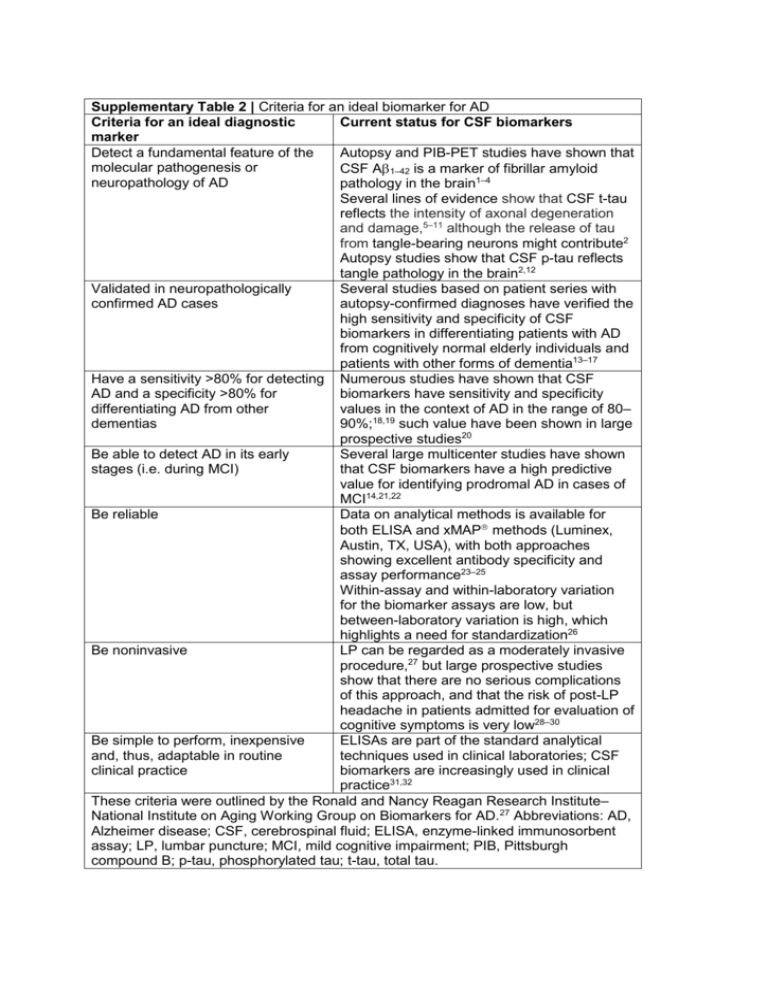
Supplementary Table 2 | Criteria for an ideal biomarker for AD Criteria for an ideal diagnostic Current status for CSF biomarkers marker Detect a fundamental feature of the Autopsy and PIB-PET studies have shown that molecular pathogenesis or CSF A 1–42 is a marker of fibrillar amyloid neuropathology of AD pathology in the brain1–4 Several lines of evidence show that CSF t-tau reflects the intensity of axonal degeneration and damage,5–11 although the release of tau from tangle-bearing neurons might contribute2 Autopsy studies show that CSF p-tau reflects tangle pathology in the brain2,12 Validated in neuropathologically Several studies based on patient series with confirmed AD cases autopsy-confirmed diagnoses have verified the high sensitivity and specificity of CSF biomarkers in differentiating patients with AD from cognitively normal elderly individuals and patients with other forms of dementia13–17 Have a sensitivity >80% for detecting Numerous studies have shown that CSF AD and a specificity >80% for biomarkers have sensitivity and specificity differentiating AD from other values in the context of AD in the range of 80– dementias 90%;18,19 such value have been shown in large prospective studies20 Be able to detect AD in its early Several large multicenter studies have shown stages (i.e. during MCI) that CSF biomarkers have a high predictive value for identifying prodromal AD in cases of MCI14,21,22 Be reliable Data on analytical methods is available for both ELISA and xMAP methods (Luminex, Austin, TX, USA), with both approaches showing excellent antibody specificity and assay performance23–25 Within-assay and within-laboratory variation for the biomarker assays are low, but between-laboratory variation is high, which highlights a need for standardization26 Be noninvasive LP can be regarded as a moderately invasive procedure,27 but large prospective studies show that there are no serious complications of this approach, and that the risk of post-LP headache in patients admitted for evaluation of cognitive symptoms is very low28–30 Be simple to perform, inexpensive ELISAs are part of the standard analytical and, thus, adaptable in routine techniques used in clinical laboratories; CSF clinical practice biomarkers are increasingly used in clinical practice31,32 These criteria were outlined by the Ronald and Nancy Reagan Research Institute– National Institute on Aging Working Group on Biomarkers for AD.27 Abbreviations: AD, Alzheimer disease; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunosorbent assay; LP, lumbar puncture; MCI, mild cognitive impairment; PIB, Pittsburgh compound B; p-tau, phosphorylated tau; t-tau, total tau. 1. Strozyk, D., Blennow, K., White, L. R. & Launer, L. J. CSF A 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 60, 652–656 (2003). 2. Tapiola, T. et al. Cerebrospinal fluid -amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch. Neurol. 66, 382–389 (2009). 3. Fagan, A. M. et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid A42 in humans. Ann. Neurol. 59, 512-519 (2006). 4. Forsberg, A. et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol. Aging 29, 1456–1465 (2008). 5. Hesse, C. et al. Transient increase in total tau but not phospho-tau in human cerebrospinal fluid after acute stroke. Neurosci. Lett. 297, 187–190 (2001). 6. Ost, M. et al. Initial CSF total tau correlates with 1-year outcome in patients with traumatic brain injury. Neurology 67, 1600–1604 (2006). 7. Zetterberg, H. et al. Neurochemical aftermath of amateur boxing. Arch. Neurol. 63, 1277– 1280 (2006). 8. Blom, E. S. et al. Rapid progression from mild cognitive impairment to Alzheimer's disease in subjects with elevated levels of tau in cerebrospinal fluid and the APOE 4/4 genotype. Dement. Geriatr. Cogn. Disord. 27, 458–464 (2009). 9. Samgard, K. et al. Cerebrospinal fluid total tau as a marker of Alzheimer's disease intensity. Int. J. Geriatr. Psychiatry (2009). 10. Wallin, A. K., Hansson, O., Blennow, K., Londos, E. & Minthon, L. Can CSF biomarkers or pre-treatment progression rate predict response to cholinesterase inhibitor treatment in Alzheimer's disease? Int. J. Geriatr. Psychiatry 24, 638–647 (2009). 11. Otto, M. et al. Elevated levels of tau-protein in cerebrospinal fluid of patients with Creutzfeldt–Jakob disease. Neurosci. Lett. 225, 210–212 (1997). 12. Buerger, K. et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain 129, 3035–3041 (2006). 13. Koopman, K. et al. Improved discrimination of autopsy-confirmed Alzheimer's disease (AD) from non-AD dementias using CSF P-tau181P. Neurochem. Int. 55, 214–218 (2009). 14. Shaw, L. M. et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann. Neurol. 65, 403–413 (2009). 15. Clark, C. M. et al. Cerebrospinal fluid tau and -amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch. Neurol. 60, 1696–1702 (2003). 16. Bian, H. et al. CSF biomarkers in frontotemporal lobar degeneration with known pathology. Neurology 70, 1827–1835 (2008). 17. Sunderland, T. et al. Decreased -amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 289, 2094–2103 (2003). 18. Blennow, K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx 1, 213–225 (2004). 19. Blennow, K. & Hampel, H. CSF markers for incipient Alzheimer's disease. Lancet Neurol. 2, 605–613 (2003). 20. Blennow, K. CSF biomarkers for Alzheimer's disease: use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 5, 661–672 (2005). 21. Mattsson, N. et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 302, 385–393 (2009). 22. Visser, P. J. et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 8, 619–627 (2009). 23. Olsson, A. et al. Simultaneous measurement of beta-amyloid(1–42), total tau, and phosphorylated tau (Thr181) in cerebrospinal fluid by the xMAP technology. Clin. Chem. 51, 336–345 (2005). 24. Vanderstichele, H. et al. Standardization of measurement of -amyloid1–42 in cerebrospinal fluid and plasma. Amyloid 7, 245–258 (2000). 25. Vanderstichele, H. et al. Analytical performance and clinical utility of the INNOTEST PHOSPHO-TAU181P assay for discrimination between Alzheimer's disease and dementia with Lewy bodies. Clin. Chem. Lab. Med. 44, 1472–1480 (2006). 26. Verwey, N. A. et al. A worldwide multicentre comparison of assays for cerebrospinal fluid biomarkers in Alzheimer's disease. Ann. Clin. Biochem. 46, 235–240 (2009). 27. [No authors listed] Consensus report of the Working Group on: "Molecular and Biochemical Markers of Alzheimer's Disease". The Ronald and Nancy Reagan Research Institute of the Alzheimer's Association and the National Institute on Aging Working Group. Neurobiol. Aging 19, 109–116 (1998). 28. Andreasen, N. et al. Evaluation of CSF-tau and CSF-A42 as diagnostic markers for Alzheimer disease in clinical practice. Arch. Neurol. 58, 373–379 (2001). 29. Blennow, K., Wallin, A. & Hager, O. Low frequency of post-lumbar puncture headache in demented patients. Acta Neurol. Scand. 88, 221–223 (1993). 30. Hansson, O. et al. Combined rCBF and CSF biomarkers predict progression from mild cognitive impairment to Alzheimer's disease. Neurobiol. Aging 30, 165–173 (2007). 31. Frisoni, G. B. et al. Markers of Alzheimer's disease in a population attending a memory clinic. Alzheimers Dement. 5, 307–317 (2009). 32. Parnetti, L., Lanari, A., Silvestrelli, G., Saggese, E. & Reboldi, P. Diagnosing prodromal Alzheimer's disease: role of CSF biochemical markers. Mech. Ageing Dev. 127, 129–132 (2006).










