Spectroscopic measurement of Rydberg`s constant for
advertisement

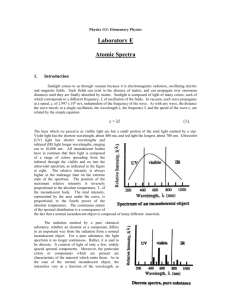
Spectroscopic measurement of Rydberg’s constant for Hydrogen H. Potter, and E. Kager (Completed 11 September 2005) Rydberg’s Constant for Hydrogen was determined to be about 11,214,870m-1, resulting in only about a 2.25% discrepancy with the presently accepted value of RH1 using a spectrometer and a diffraction grating. I. Introduction Long before Bohr developed his orbital model of the atom and derived the general equation 1/λ = RH(1/(nf2)-1/(ni2)) (1) relating the wavelength of the light emitted by an excited atom to its orbital transition, Balmer had already determined that a similar equation, 1/λ = k((1/4)-1/(m2)), (2) where m is an integer and k is a constant, accurately described the spectroscopic lines when visible light was emitted. Balmer was able to come up with his formula empirically due to the abundance of data available for visible emissions. The scattering from a set of Bragg planes establishes the Bragg condition for constructive interference, referred to in the context of this experiment as the grating equation, that nλ = 2dsin(θ), (3) where n is the order of the observed diffraction, λ is the wavelength of the incident light, d is the spacing of the diffraction grating, and θ is the angle through which the light is diffracted. II. Experiment The ease of measurement of diffractions in the visible spectrum due to the ability of the human eye to observe them was utilized in this experiment. The visible spectra emitted by excited sodium and hydrogen atoms were observed using a spectrometer in order to determine the diffraction angles of each atom’s various emissions in both the first and second order through a diffraction grating. For the sodium, the presently accepted values for the wavelengths of the sodium emissions2 were taken as known quantities. This enabled the calculation of the spacing in the diffraction grating using the grating equation (3). The mean of the calculated values for the spacing of the diffraction grating was taken to be the spacing of the diffraction grating for the remainder of the experiment. For the hydrogen, the wavelengths of the various spectral lines were calculated for each measurement using the grating equation (3). Four spectral lines were observed. The diffraction angles for the red, turquoise, and light purple spectral lines were observed and recorded in both the first and second order as they bent both to the left and right side of the spectrometer. The diffraction angles for the dark purple line, however, could only be recorded in the first order as it bent to the left and right side of the spectrometer. Using Bohr’s orbital transition model for atomic emissions each observed value of λ could reasonably be paired with its orbital transition. The orbital transitions for each spectral line were always to the second orbital because if they were not they would not have been observable in the visible spectrum. The orbital from which this transition was made was different for each spectral line, increasing incrementally from 3 to 6 as the color changed from red to dark purple. This was justified because a more energetic emission requires a larger orbital transition, and light has more energy at shorter wavelengths. This pairing led to14 data points of the form ((1/(nf2)-1/(ni2)), 1/λ). Comparing this plot to equation (1), it is easy to see that, since equation (1) is linear with regards to the quantities being plotted, a linear regression on these data points should yield a line with slope RH. The value of RH thus obtained was taken to be the experimentally calculated value of RH. III. Results Sodium Data: n = Order of Line θ n 1 2 1 2 20.9 46.0 21.1 45.9 Accepted λ (m) 2 5.896E-07 5.896E-07 5.890E-07 5.890E-07 Mean: θ = Measured Angle Grating Equation: 2dsin(θ) = nλ d (m) Uncertainty in d 8.26E-07 1.35E-09 8.20E-07 9.94E-10 8.18E-07 1.33E-09 8.20E-07 9.96E-10 8.21E-07 1.17E-09 Table 1: Data obtained for spectral lines of sodium that was used to calculate the spacing of the diffraction grating. The uncertainty in d was calculated using error propagation analysis by assuming the uncertainty in all quantities but the angle measurement were negligible. 2 Hydrogen Data: Left = Light Shield Right = No Light Shield Color Dark Purple Dark Purple Light Purple Light Purple Light Purple Light Purple Turquoise Turquoise Turquoise Turquoise Red Red Red Red Side Left Right Left Right Left Right Left Right Left Right Left Right Left Right n 1 1 1 1 2 2 1 1 2 2 1 1 2 2 Balmer's Formula: 1/λ = RH(1/(nf2) - 1/(ni2)) Use Mean d From Above λ = wavelength θ 14.3 14.3 15.2 15.0 31.7 31.5 17.0 17.0 36.4 35.9 23.5 23.2 53.0 52.5 "x" "y" -1 n f n i 1/(n f 2 ) - 1/(n i 2 ) 1/λ (m ) Uncertainty in λ Uncertainty in "y" λ (m) 4.06E-07 2 6 0.2222 2465474 3.35E-09 2.04E+04 4.06E-07 2 6 0.2222 2465474 3.35E-09 2.04E+04 4.31E-07 2 5 0.2100 2322635 3.38E-09 1.82E+04 4.25E-07 2 5 0.2100 2352878 3.37E-09 1.87E+04 4.31E-07 2 5 0.2100 2317802 1.83E-09 9.85E+03 4.29E-07 2 5 0.2100 2330991 1.83E-09 9.95E+03 4.80E-07 2 4 0.1875 2082861 3.42E-09 1.49E+04 4.80E-07 2 4 0.1875 2082861 3.42E-09 1.49E+04 4.87E-07 2 4 0.1875 2052411 1.85E-09 7.78E+03 4.81E-07 2 4 0.1875 2077075 1.85E-09 7.96E+03 6.55E-07 2 3 0.1389 1527200 3.56E-09 8.30E+03 6.47E-07 2 3 0.1389 1545836 3.55E-09 8.49E+03 6.56E-07 2 3 0.1389 1525025 1.79E-09 4.17E+03 6.51E-07 2 3 0.1389 1535179 1.80E-09 4.24E+03 Table 2: Data obtained for spectral lines of hydrogen that was used to calculate λ values and to create a linear plot of Balmer’s Formula (1) with the experimental data. The uncertainties in both λ and in “y” were calculated using error propagation. IV. Analysis and Discussion Linear Plot 1/Lambda m -1 2.5E+06 2.3E+06 y = 11,214,870x - 23,269 R2 = 0.999 2.1E+06 1.9E+06 1.7E+06 1.5E+06 0.13 0.18 0.23 1/4 - 1/(n*n) Figure 1: Linear plot of the hydrogen data according to Balmer’s Formula (1). The slope of the line obtained via linear regression is the experimentally obtained value of RH. Using a least-squares linear regression on the 14 data points, the linear fit shown in Figure 1 was obtained. The slope of the best-fit line is 11,214,870m-1, compared with the accepted value1 for RH of 10,967,760m-1, an absolute discrepancy of 247,110m-1, but a percentage discrepancy of only 2.25%. The linear fit was also fantastic, with r2 = .9990 and correlation coefficient r = .9995. 3 The linear fit, however, has a nonzero additive constant of -23,269m-1. Fortunately, in analogy with the absolute discrepancy, this is small relative to the enormity of the slope, being only about 0.2% as large. The uncertainty in the measurement of diffraction angles was only .1˚; however, since the measurement of the wavelength of the light in each case was dependent upon both the diffraction grating spacing and the diffraction angle, and the measurement of the diffraction grating spacing was also dependent upon the angle of diffraction, the uncertainty in the wavelength of the light in each case was doubly dependent upon the uncertainty in the angle. Such error propagation analysis enabled error bars to be included on the linear plot. This allowed a maximum and minimum value of RH to be estimated by calculating a maximum and minimum reasonable slope of the linear fit. Taking points (5/36, 1500000) and (2/9, 2500000), and (5/36, 1600000) and (2/9, 2400000) leads to RHmax = 12,000,000m-1 and RHmin = 9,600,000m-1. V. Conclusion The calculated value for RH was within the specified experimental uncertainty of the accepted value1; however, several avenues are available for improving the precision of this experiment. One source of uncertainty was the measurement of each angle when reading the Vernier scale on the spectrometer. The telescope of the interferometer tended to interfere with a precisely vertical reading of the Vernier scale, and the eye strain resulting from finding and centering the spectral lines in the eyepiece compounded the difficulty. A finely calibrated digital display would alleviate these problems. A method for focusing the spectrometer automatically and with less uncertainty would also make the determination of the center of each of the spectral lines in the eyepiece more precise. Conducting the experiment in a pitch black dark room would also ease the process of finding and centering spectral lines, possibly enabling the detection of the second order dark purple line, though data collection would be impeded in such a situation. 1 Tipler, Paul A. and Ralph A. Llewellyn, Modern Physics, 3rd ed. (W. H. Freeman and Company, New York 2003), p.164. 2 CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data, edited by Robert C. Weast (CRC Press, Cleveland, OH, 1975), p.E-213. 4