The Translational Research Cycle – Example in Infectious Diseases
advertisement

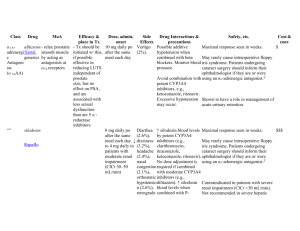
The Translational Research Cycle – Example in Infectious Diseases Topic: Adverse Drug-Drug Interactions in Treating Infectious Diseases Co-infection with different organisms is a common problem in infectious diseases therapy. This is especially true in HIV-AIDS populations, where immune suppression greatly increases opportunistic infections from other organisms, such as tuberculosis (TB). In many parts of the developing world, TB is the most common opportunistic infection in HIV-AIDS patients. For example, it has been estimated that 30% of HIVAIDS patients are co-infected with TB world wide (UNAIDS). Although different drugs are used to treat each disease, in some instances the administration of one drug greatly alters the efficacy of the other. Translational research cycle T0 – Basic laboratory ‘discovery’ of a new mechanism for preventing certain adverse drug-drug interactions. The isothiocyanate, sulforaphane (SFN), is a common dietary constituent of broccoli and broccoli sprouts. It has been extensively studied as a putative ‘chemopreventive’ agent, potentially reducing the incidence of cancer via several pathways, including ‘up regulation’ of genes that code for ‘antioxidant’ pathways. In the process of studying how SFN alters gene expression in human liver cells in primary culture, a ‘microarray’ experiment evaluated the effects of SFN on gene expression of ~20,000 genes. It was noted that the mRNA levels for one particular gene, Cytochrome P450 3A4 (CYP3A4), was largely absent. CYP3A4 is normally expressed at relatively high levels in liver and small intestine, and plays a major role in metabolism of many drugs and non-drug chemicals. CYP3A4 is ‘inducible’ by many drugs and dietary constituents, but there were few, if any, studies showing how substances could ‘down regulate’ (inhibit gene expression) of CYP3A4, although it was known that some dietary constituents, such as grapefruit juice, could inhibit the enzymatic activity of CYP3A4. Thus, this observation led to additional studies to explore the molecular mechanism by which SFN inhibited the expression of CYP3A4 mRNA in human liver cells. CTRI interaction Translational Technologies and Resources Core (TTRC) – Center for array technologies T1 –In vitro laboratory studies to establish the molecular mechanism by which SFN inhibits CYP3A4 gene expression. Based on the initial observation described above, additional laboratory studies were done to evaluate the mechanism(s) by which SFN inhibited CYP3A4 gene expression in human liver cells. Through a series of in vitro studies, it was demonstrated that SFN binds to, but does not activate, a nuclear receptor called the ‘Pregnane X-Receptor’ (PXR, also called SXR or NR1I2). SFN appeared to tightly, perhaps irreversibly, bind to the ligand binding site of the receptor, thus preventing other ligands from binding and activating the receptor. Ligand activation of PXR was known to be a major contributor to both ‘constitutive’ (basal) and inducible expression of CYP3A4. Thus, the apparent molecular mechanism by which SFN blocks CYP3A4 gene expression in human hepatocytes is by inhibiting ligand activation of PXR. This has immediate clinical relevance to adverse drug interactions, because it is known that many drugs act as ligands for PXR, thereby inducing expression of CYP3A4, which then enhances the clearance of other drugs that happen to be substrates for CYP3A4. The best example of this is the anti-tuberculosis drug, rifampin. Rifampin is a potent activating ligand for PXR, and increases the activity of CYP3A4 many fold. For example, a normal therapeutic regiment with rifampin TTR/CSR cores. Several Technology Access Facilities identified by the TTRC could be accessed, such as Molecular Modeling, Proteomics, and pharmacokinetics/drug metabolism Mass Spectrometry facility. Appropriate for Technology Access Grant via CSR. CORT and CBS – epidemiologic studies documenting ARV treatment failure with use will increase the clearance of the sedative, midazolam, by 10-fold or more. Midazolam is cleared almost exclusively through metabolism via CYP3A4. Most first-line antiretroviral (ARV) drugs, including the non-nucleoside reverse transcriptase inhibitor nevirapine and the protease inhibitor lopinavir/ritonavir are also substrates for CYP3A4. Thus, ARV treatment options are limited when TB therapy with rifampin is required because it will induce CYP3A4, thereby increasing clearance and reducing the efficacy of the ARVs. Although some new drugs have become available (at least in developed countries) to work around this adverse drug-drug interaction, it remains a significant public health problem in developing countries where co-infection with TB and HIV-AIDS are common, and both rifampin and first generation ARV drugs are widely used for TB and HIVAIDS therapy, respectively. Based on these basic laboratory discoveries, a ‘use patent’ was filed to protect the ‘intellectual property’ related to the use of isothiocyanates to block ligand activation of PXR and subsequent changes in gene expression. of rifampin or other antiTB drugs T2 - Clinical investigations of Sulforaphane as a clinically effective antagonist to ligand activation of PXR. A phase I, ‘proof of principle’ clinical trial was funded by NIH to test the hypothesis that co-administration of SFN with rifampin would block rifampin-mediated induction of CYP3A4, measured by assessment of midazolam clearance before and after 7 days of treatment with rifampin. The study is being performed in the CTRI CRC, via collaboration between the UW CRC and FHCRC Prevention Center. The design is an open label, cross-over study with 3 arms – 1) sulforaphane alone, 2) rifampin alone, and 3) sulforaphane plus rifampin. Twenty four healthy volunteers who meet study requirements receive 7 days of treatment for each arm, with at least two weeks between each arm to allow for a return to equilibrium of CYP3A4 activity. CYP3A4 activity was assessed prior to and following the 7 day treatment regiment by pharmacokinetic analysis of midazolam ‘AUC’. Previous clinical studies with rifampin and midazolam have demonstrated that rifampin treatment will reduce the AUC of midazolam by 90-95%. If the proof of principle study demonstrates significant efficacy of SFN in preventing rifampin-mediated increase in midazolam clearance, then further clinical studies would be warranted. IND submission support (Pre-clinical core) and IRB submission, regulatory consultation (RSB), protocol development and database systems (CBS/BMI), potential pilot funding (CSR), conduct the study (CRCN) and research coordinators for implementation (RSB). Based on positive results in the early Phase I clinical trial discussed above, a trial in normal, healthy volunteers will be conducted to demonstrate that coadministration of SFN with rifampin will prevent the enhanced clearance of ritonavir normally associated with rifampin treatment. The design would be similar to the Phase I study above, but would look directly at clearance of ritonavir. A further Phase II trial investigating the rifampin/SFN interaction with other ARVs would then be conducted in HIV-infected patients taking a variety of ARV medications. HIV-infected adults on a stable ARV regimen including either a protease inhibitor (PI) or a non-nucleoside reverse transcriptase inhibitor (NNRTI) would participate in an open-label study of the effect of rifampin/SFN administration on the pharmacokinetics of the PI or NNRTI. If these Phase II clinical trials proved successful, a Phase III clinical trial would then be designed to determine if SFN co-formulation with rifampin is effective in preventing the rifampin – ARV interaction, and does not alter efficacy of either drug in the treatment of their targeted diseases. (Prior to this step could be a return to T1 activities to develop rifampin/SFN co-formulated into a single Entrepreneurial Law Clinic, pre-clinical research consulting Preclinical core’s drug and device consultation group. RSB, BMI and CBS’s assistance in setting up a clinical development plan and databases to follow and track the data. Continued input from RSB, CBS, preclinical cores with the PI to review the initial results and decide on the next steps. Can the project move forward or must there be more preclinical formulation work? The development of a Phase 3 protocol will require multiple cores, RSB for IRB applications, IND and regulatory issues and bioethics issues that may arise, CBS/BMI for protocol development, CRCN for study conduct, CORT for use of practice tablet/capsule.) T3- Dissemination and implementation of SFN co-formulations to prevent drug-drug interactions. If the Phase III trial confirms the efficacy of the rifampin/SFN co-formulation in mitigating the rifampin-ARV drug-drug interaction, thus allowing the concurrent treatment of TB and HIV, further work would be done to revise guidelines. In addition, further studies would be warranted investigating the use of SFN in other clinical situations where significant drug interactions due to drug metabolism via the CYP3A4 enzymatic pathway confound optimal therapy. T4 – Evaluation of the public health impact of preventing adverse drug-drug interactions via antagonism of PXR activation. Further work would be done to evaluate the uptake of the revised TB-HIV treatment guidelines and investigate drug distribution to the areas of the world most affected by the TB-HIV epidemic and the effects on TB-HIV mortality. based networks for study conduct, TTRC for potential assay development for clinical trial safety or efficacy endpoints, Preclinical for industry partnerships to financially support and potentially run such a trial. Collaboration with external groups such as the Center for AIDS Research CORT for work through the practice based networks to study utilization of therapy working with CBS/BMI. Bioethics CORT, BMI, CBS