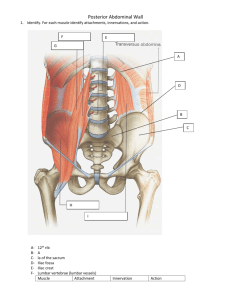
Diaphragmatic/Phrenic Nerve Stimulation MED.00100
REVIEW REQUEST FOR
Diaphragmatic/Phrenic Nerve Stimulation &
Diaphragm Pacing Systems
Provider Data Collection Tool Based on Medical Policy MED.00100
Policy Last Review Date: 02/05/2015 Policy Effective Date: 04/07/2015
Individual’s Name:
Insurance Identification Number:
Provider Tool Effective Date: 04/07/2015
Date of Birth:
Individual’s Phone Number:
Provider ID Number: Ordering Provider Name & Specialty:
Office Address:
Office Phone Number: Office Fax Number:
Rendering Provider Name & Specialty:
Office Address:
Office Phone Number:
Provider ID Number:
Office Fax Number:
Facility Name:
Facility Address:
Date/Date Range of Service:
Service Requested (CPT if known):
Diagnosis Code(s) (if known):
Facility ID Number:
Place of Service: Home Inpatient
Outpatient Other:
Please check all that apply to the individual:
Complete the form in its entirety to facilitate your requested review .
I. Diaphragmatic/Phrenic Stimulation
Request is for: (Check all that apply):
FDA-approved diaphragmatic stimulation device
FDA-approved phrenic nerve stimulation device
Clinical Criteria (check all that apply):
Device is to be used as an alternative to invasive mechanical ventilation
Individual is less than 18 years of age
Individual is 18 years of age or older
Individual has ventilatory failure due to a stable high spinal cord injury
Individual cannot breathe spontaneously for 4 continuous hours or more without use of a mechanical ventilator;
Individual has ventilatory failure due to central alveolar hypoventilation syndrome
Individual has temporary respiratory insufficiency
Individual has a condition with intact phrenic nerve and diaphragm function (for example, chronic obstructive lung disease,
restrictive lung disease, singultus [hiccups])
Other (Please list):
Page 1 of 3
REVIEW REQUEST FOR
Diaphragmatic/Phrenic Nerve Stimulation &
Diaphragm Pacing Systems
Provider Data Collection Tool Based on Medical Policy MED.00100
Policy Last Review Date: 02/05/2015 Policy Effective Date: 04/07/2015 Provider Tool Effective Date: 04/07/2015
Individual has diaphragm movement with stimulation visible under fluoroscopy
Stimulation of the diaphragm either directly or through the phrenic nerve resulting in sufficient muscle activity to
accommodate independent breathing without the support of a ventilator for at least 4 continuous hours a day
Individual has normal chest anatomy, a normal level of consciousness and the ability to participate in and complete the
training and rehabilitation associated with the use of the device
Individual has bilateral clinically acceptable phrenic nerve function that is demonstrated with EMG recordings and nerve
conduction times
Individual has the ability to breathe spontaneously for 4 continuous hours or more without use of a mechanical ventilator
Individual has underlying cardiac, pulmonary or chest wall disease which is significant enough to prevent spontaneous
breathing off a ventilator for more than 4 hours even with the use of the phrenic nerve or diaphragm pacemaker device.
Other (Please list):
II. Diaphragmatic Stimulation
Request is for: (Check all that apply):
Diaphragm stimulation with an FDA approved diaphragm pacing system
Clinical Criteria (check all that apply):
Device to be used as an alternative to invasive mechanical ventilation
Individual is less than 18 years of age
Individual is 18 years of age or older
Individual has ventilatory failure due to a stable high spinal cord injury
Individual has ventilatory failure due to central alveolar hypoventilation syndrome
Individual has ventilatory failure due to motor neuron disease, for example amyotrophic lateral sclerosis (ALS)
Individual cannot breathe spontaneously for 4 continuous hours or more without use of a mechanical ventilator;
Individual has temporary respiratory insufficiency
Individual has a condition with intact phrenic nerve and diaphragm function (for example, chronic obstructive lung disease,
restrictive lung disease, singultus [hiccups])
Other (Please list):
Individual has diaphragm movement with stimulation visible under fluoroscopy
Stimulation of the diaphragm directly results in sufficient muscle activity to accommodate
independent breathing without the support of a ventilator for at least 4 continuous hours a day;
Individual has normal chest anatomy, a normal level of consciousness and has the ability to participate in and complete the
training and rehabilitation associated with the use of the device
Individual has the ability to breathe spontaneously for 4 continuous hours or more without use of a mechanical ventilator
Individual has underlying cardiac, pulmonary or chest wall disease which is significant enough to prevent spontaneous
breathing off a ventilator for more than 4 hours even with the use of the phrenic nerve or diaphragm pacemaker device.
Other (Please list):
Page 2 of 3
REVIEW REQUEST FOR
Diaphragmatic/Phrenic Nerve Stimulation &
Diaphragm Pacing Systems
Provider Data Collection Tool Based on Medical Policy MED.00100
Policy Last Review Date: 02/05/2015 Policy Effective Date: 04/07/2015 Provider Tool Effective Date: 04/07/2015
This request is being submitted:
Pre-Claim
Post–Claim. If checked, please attach the claim or indicate the claim number
I attest the information provided is true and accurate to the best of my knowledge. I understand that the health plan or its designees may perform a routine audit and request the medical documentation to verify the accuracy of the information reported on this form.
Name and Title of Provider or Provider Representative Completing
Form and Attestation (Please Print)*
Date
*The attestation fields must be completed by a provider or provider representative in order for the tool to be accepted.
Anthem UM Services, Inc., a separate company, is the licensed utilization review agent that performs utilization management services on behalf of your health benefit plan or the administrator of your health benefit plan.
Page 3 of 3