Supplementary Table 1
advertisement

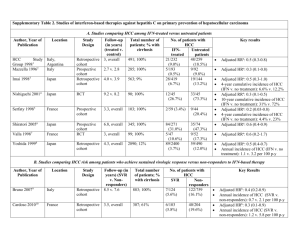
Supplementary Table 1 | Immunotherapies with FDA-approval for cancer treatment Agent Class Indication FDA Ipilimumab Anti-CTLA-4-blocking mAb Metastatic melanoma Reference ID: 3417,736 Nivolumab Anti-PD-1-blocking mAb Metastatic melanoma, squamous lung cancer Reference ID: 3677,021 (2nd line) Pembrolizumab Anti-PD-1-blocking mAb Metastatic melanoma Reference ID: 3621,876 Sipuleucel Cell-based vaccine Castration resistant metastatic prostate cancer SR-1346.02, 28 SEP (Provenge) High dose IL-2 2009 Cytokine Metastatic melanoma, metastatic renal cell cancer Abbreviations: CTLA-4, cytotoxic T-lymphocyte protein 4; mAb, monoclonal antibody. 1 Reference ID: 3165,255 Supplementary Table 2 | Clinical trials on cell-based immunotherapies (DCs) in HCC Reference Agent Treatment schedule Study design Population Endpoint Relevant findings Chi et al. Autologous 2 IT injections Prospective, 14 patients with Not reported* RR: 2 PR, 7 SD by WHO criteria; (2005)S1 immature DCs 2 days after EBRT single arm, advanced tumours increased AFP-specific immune (8 Gy) separated by dose finding suitable for EBRT responses in 4/10 patients; 21 days increased NK cell activity in 3/10 patients; no relevant toxicity Nakamoto Autologous 1 IA injection Prospective, 10 patients with HCV- Not reported* No difference in RFS compared with et al. immature DCs mixed with single arm related tumours in a historical control group; DC (2007)S2 pulsed with Gelfoam, during TNM stage >II treated migration to regional lymph nodes HepG2-derived selective with TAE not observed; ELISPOT response to tumour lysate embolization AFP, hTERT, Her-2/neu and MRP3 increased by 2–20-fold in 6/8 patients; no relevant toxicity Butterfield Autologous 3 biweekly ID Prospective, 16 HLA-A*0201 Safety and No AFP or tumour responses; et al. immature DCs injections single arm, patients with AFP- immunological substantial but mostly modest (2006)S3 pulsed separately dose finding expressing TNM stage III effects* tetramer responses in 6/10 patients with 4 AFP and IV tumours with concordant ELISPOT responses peptides in 5/6; enhanced NK cell activation; inability to generate acceptable DCs in 2 patients; no AEs attributable to vaccination Tada et al. Autologous 6 SC injection and Prospective, 5 patients with early Safety, feasibility RR: No tumour response; strong (2012)S4 mature DCs topical imiquimod single arm and intermediate and immune T-cell responses against HCC pulsed with three every 2 weeks after tumours with no prior activity* antigens in 5/5 patients tumour- TACE response to TACE (particularly against AFP and associated MAGE-1); no relevant toxicity proteins AFP, MAGEA1, GPC3 Mazzolini Autologous 3 IT injections on Prospective, 17 patients with 2 P: feasibility and RR: 0 PR, 1 SD/8 HCC pts; 1/8 et al. mature DCs metastatic digestive safety* positive DTH with tumour lysate in (2005)S5 transduced with tumours (8 patients S: biological effect HCC patients; substantial increase an adenoviral with advanced HCC) and antitumour in tumour infiltration by effector activity immune cells in 5 patients with weeks 0, 3 and 6 single arm vector encoding human IL-12 HCC; no relevant toxicity (no DLT) Qiu et al. Autologous 2 to 7 IV weekly Prospective, 9 patients with stage III (2011)S6 mature DCs injections (no single arm tumours treated by positive DTH after Tx; prolonged pulsed with prespecified cytoreductive resection survival compared to a historical tumour lysate schedule reported) followed by RFA, control group (median 17.1 versus incubated with chemotherapy or 10.1 months); increased frequency of recombinant interventional Tx IFN-γ-producing T cells; fever and Not defined* bovine α1,3GT, RR: 3 PR; 0 SD; 8/9 patients had rash common but not severe cocultured with BM-derived CIKs El Ansary Autologous Prospective, 30 patients with et al. mature DCs 1 ID injection randomized advanced HCC not reported); increased peripheral CD8+ (2013)S7 pulsed with (BSC) amenable to surgery, T cells; no serious AEs reported HepG2-derived Not defined* RR: 2 PR; 9 SD (criteria not local ablation or TACE tumour lysate Palmer Autologous one to six three- Prospective, 39 patients with P: tumour response, RR: 1 PR, 6 SD/25 evaluable et al. matured DCs weekly IV single arm advanced HCC not toxicity patients; 4 /17 patients had AFP (2009)S8 pulsed with injections amenable to surgery, S: changes in serum responses; no relevant toxicity local ablation or TACE. AFP and immune including hepatic toxicity or response de novo autoantibody formation Not reported* RR: 4 PR, 17 SD/31 patients HepG2-derived tumour lysate Lee et al. Autologous Group 1: 5 weekly Prospective, 31 patients with TNM (2005)S9 mature DCs IV injections; group single arm stage IV tumours pulsed with 2: same plus increased survival in comparison autologous monthly injections with a historical control group; no tumour lysate for 2–12 months relevant toxicity evaluable; 0/10 DTH responses; *The method used for sample size calculation was not reported. Abbreviations: α1,3GT, α1,3-galactosyltransferase; AE, adverse events; AFP, α-fetoprotein; BSC, best supportive care; CIK, cytokine-induced killer cells; DC, dendritic cell; DLT, dose-limiting toxicity; DTH, skin delayed hypersensitivity test; EBRT, 3 external beam radiation therapy; ELISPOT, enzyme-linked immunospot; GPC3, glypican-3; Gy, grey; HCC, hepatocellular carcinoma; Her-2/neu, receptor tyrosine-protein kinase erbB-2; hTERT, telomerase reverse transcriptase; IA, intra-arterial; ID, intradermal; IT, intrathecal; IV, intravenous; MAGEA1, melanoma-associated antigen 1; MRP3, canalicular multispecific organic anion transporter 2; NK, natural killer; P, primary endpoint; PR, partial response; RFA, radiofrequency ablation; RFS, recurrence-free survival; RR, response rate; S, secondary endpoint; SC, subcutaneous; SD, stable disease; TNM, tumour-node-metastasis; TACE, transarterial chemoembolization; TAE, transarterial embolization; Tx, treatment. 4 Supplementary Table 3 | Clinical trials on cell-based immunotherapies (CIKs) in HCC Reference Agent Treatment schedule Study design Population Endpoint Relevant findings Takayama Autologous 6 IV infusions 2 to Prospective, 150 patients P: 3-year RFS and Increased 3-year RFS (37% versus et al. lymphocytes 24 months after randomized undergoing R0 TTR 22%) and TTR (median: 2.8 versus (2000)S10 activated with resection resection (76 received S: disease-specific 1.6 years); no difference in OS; no CIKs) survival and OS relevant toxicity Clinical effect No difference in recurrence rates; rIL-2 Hui et al. Autologous 3 (group 1) or 6 Prospective, 127 patients with (2009)S11 lymphocytes (group 2) IV randomized solitary tumours treated increase in RFS (5-year rate: 23%, activated with infusions every (BSC); sample by R0 resection (84 19% and 11% in groups 1, 2 and rIL-2 and rIL-1a 2 weeks after size received CIKs) control); no difference in OS; 5 resection calculation patients had DLT (persistent fever) not reported Pan et al. Autologous At least 4 IV (2013)S12 lymphocytes injections every solitary resected (5-year OS: 65.9% versus 50.2%); activated with 2 weeks after tumours (no selection multivariate survival analysis rIL-2 and rIL-1a resection criteria for CIK showed AST, tumour size, tumour therapy) compared number, pathological grades and with a historical CIK Tx as independent prognostic Retrospective 204 patients with OS cohort Increased OS in CIK-treated patients factors Weng et al. Autologous 8–10 IV injections Prospective, 45 patients with (2008)S13 lymphocytes given every randomized nodular or massive RFS rate (9% versus 30%); increase activated with 15 days, starting (BSC) HCC (2–13 cm) in in circulating CD3+, CD4+, and rIL-2 6–8 weeks after vascular complete CD3+CD56+ cells and decreased CD8+ locoregional response after TACE T cells; no relevant toxicity therapy and RFA Tx P: TTR and RFS TTR not reported; reduced 1-year Ma et al. Autologous 6 IV infusions Prospective, 7 patients with small P: changes in Increased proportion of CD3+CD8+ (2010)S14 lymphocytes every 2 weeks single arm (1.0–3.5 cm) tumours; peripheral cell T cells (36% versus 48.%); increased activated with within 3 months tumour-naive or subsets and IFN-γ blood levels of IFN-γ (7.6 versus rIL-2, and after RFA relapsing after prior levels; tumour 4.7 pg/ml); no recurrence after therapy recurrence 7 month follow-up RetroNectin® 5 (Takara Bio Inc., Japan) Yu et al. Autologous 2 IV monthly until Prospective, 132 patients with early P: OS Increased OS in CIK-treated patients (2014)S15 lymphocytes recurrence (post- randomized to advanced tumours S: PFS (median 24.9 versus 11.3 months); activated with resection), (BSC) receiving standard Tx no difference in recurrence rate or rIL-2 and rIL-1a progression (post- (66 received CIKs) pattern after resection; no relevant TACE or BSC) or toxicity up to 36 months Shi et al. Autologous 3 IV infusions 10 Prospective, 13 patients with mostly P: Changes in Increased proportion of CD3+CD8+, (2004)S16 lymphocytes to 15 days after single arm advanced HBV-related peripheral CD3+CD56+ and CD25+ cells and activated with apheresis tumours lymphocytes subsets type I and II dendritic cells rIL-2 Abbreviations: AST, aspartate aminotransferase; BSC, best supportive care; CIK, cytokine-induced killer cells; DLT, dose-limiting toxicity; HCC, hepatocellular carcinoma; IV, intravenous; OS, overall survival; P, primary endpoint; PFS, progression free survival; R0, negative-margin resection; RFA, radiofrequency ablation; RFS, recurrence-free survival; S, secondary endpoint; TACE, transarterial chemoembolization; TTR, time to recurrence; Tx, treatment. 6 Supplementary reference list S1. Chi, K. H. et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J. Immunother. 28, 129–135 (2005). S2. Nakamoto, Y. et al. Combined therapy of transcatheter hepatic arterial embolization with intratumoral dendritic cell infusion for hepatocellular carcinoma: clinical safety. Clin. Exp. Immunol. 147, 296–305 (2007). S3. Butterfield, L. H. et al. A Phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four αfetoprotein peptides. Clin. Cancer Res. 12, 2817–2825 (2006). S4. Tada, F. et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 41, 1601–1609 (2012). S5. Mazzolini, G. et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 23, 999–1010 S6. Qiu, Y. et al. Hepatocellular carcinoma-specific immunotherapy with synthesized α1,3- galactosyl epitope-pulsed dendritic cells and cytokineinduced killer cells. World J. Gastroenterol. 17, 5260–5266 (2011). S7. El Ansary, M. et al. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J. Cancer Res. Clin. Oncol. 139, 39– 48 (2013). S8. Palmer, D. H. et al. A Phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49, 124–132 (2009). S9. Lee, W. C. et al. Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J. Immunother. 28, 496–504 (2005). S10. Takayama, T. et al. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 356, 802–807 (2000). S11. Hui, D., Qiang, L., Jian, W., Ti, Z. & Da-Lu, K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig. Liver Dis. 41, 36–41 (2009). S12. Pan, K. et al. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann. Surg. Oncol. 20, 4305–4311 (2013). S13. Weng, D. S. et al. Minimally invasive treatment combined with cytokine-induced killer cells therapy lower the short-term recurrence rates of hepatocellular carcinomas. J. Immunother. 31, 63–71 (2008). S14. Ma, H. et al. Therapeutic safety and effects of adjuvant autologous RetroNectin activated killer cell immunotherapy for patients with primary hepatocellular carcinoma after radiofrequency ablation. Cancer. Biol. Ther. 9, 903–907 (2010). S15. Yu, X. et al. A randomized Phase II study of autologous cytokine-induced killer cells in treatment of hepatocellular carcinoma. J. Clin. 7 Immunol. 34, 194–203 (2014). S16. Shi, M. et al. Autologous cytokine-induced killer cell therapy in clinical trial Phase I is safe in patients with primary hepatocellular carcinoma. World J. Gastroenterol. 10, 1146–1151 (2004). 8