Tool for Assessing a Nosocomail Infection Surveiallnce Program*
advertisement
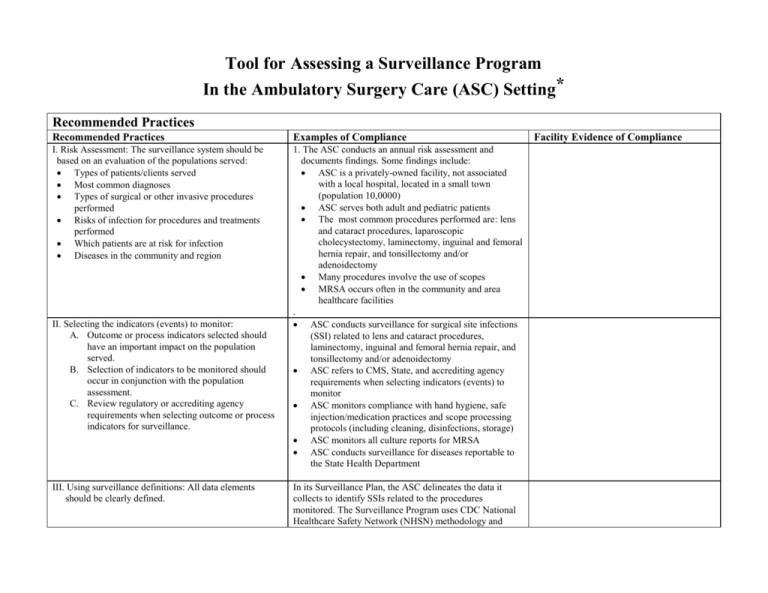
Tool for Assessing a Surveillance Program In the Ambulatory Surgery Care (ASC) Setting* Recommended Practices Recommended Practices Examples of Compliance I. Risk Assessment: The surveillance system should be based on an evaluation of the populations served: Types of patients/clients served Most common diagnoses Types of surgical or other invasive procedures performed Risks of infection for procedures and treatments performed Which patients are at risk for infection Diseases in the community and region 1. The ASC conducts an annual risk assessment and documents findings. Some findings include: ASC is a privately-owned facility, not associated with a local hospital, located in a small town (population 10,0000) ASC serves both adult and pediatric patients The most common procedures performed are: lens and cataract procedures, laparoscopic cholecystectomy, laminectomy, inguinal and femoral hernia repair, and tonsillectomy and/or adenoidectomy Many procedures involve the use of scopes MRSA occurs often in the community and area healthcare facilities . ASC conducts surveillance for surgical site infections (SSI) related to lens and cataract procedures, laminectomy, inguinal and femoral hernia repair, and tonsillectomy and/or adenoidectomy ASC refers to CMS, State, and accrediting agency requirements when selecting indicators (events) to monitor ASC monitors compliance with hand hygiene, safe injection/medication practices and scope processing protocols (including cleaning, disinfections, storage) ASC monitors all culture reports for MRSA ASC conducts surveillance for diseases reportable to the State Health Department II. Selecting the indicators (events) to monitor: A. Outcome or process indicators selected should have an important impact on the population served. B. Selection of indicators to be monitored should occur in conjunction with the population assessment. C. Review regulatory or accrediting agency requirements when selecting outcome or process indicators for surveillance. III. Using surveillance definitions: All data elements should be clearly defined. In its Surveillance Plan, the ASC delineates the data it collects to identify SSIs related to the procedures monitored. The Surveillance Program uses CDC National Healthcare Safety Network (NHSN) methodology and Facility Evidence of Compliance Recommended Practices Recommended Practices Examples of Compliance surveillance definitions as criteria for categorizing an infection.1 IV. Collecting surveillance data: A. Data collection process should be managed by knowledgeable professionals. B. Data should be collected consistently. C. Appropriate information sources should be available. D. Appropriate tools should be used to collect data. E. Appropriate post-discharge surveillance should be used. The Infection Preventionist (IP) has attended an infection prevention and control training course that included instructions on how to conduct surveillance. Surveillance data has been collected by a trained IP using the same methodology for over 12 months. A standardized data collection form is used for each surveillance indicator. Laboratory reports, physician reports, patient charts, and electronic medical records are used when collecting surveillance data. The IP uses a personal computer to collect, store and manage data. ASC surgeons are required to complete a post discharge SSI surveillance program form and return to IC department The same person collects surveillance data using a standardized data collection form. If that person is out of work for a prolonged period (such as a 2-week vacation), a mechanism is developed to obtain accurate surveillance data during that period. V. Calculating and analyzing data: A. Rates should be used when numerically measuring an outcome or process. B. Appropriate calculations should be used and reported. C. Consistent methodology should be used over time. D. All aspects of surveillance must be equivalent in order to compare rates over time within an institution or between institutions. VI. Applying risk stratification methodology: A risk stratification method should be used. For some rates, risk stratification is not possible. If rates are stratified, assure that subpopulation sizes are large enough to be statistically meaningful CDC NHSN risk stratification is used for SSI surveillance1 (i.e., a record on every patient undergoing a monitored NHSN operative procedure is generated that includes the data required in the NHSN Manual) Surveillance program uses rates (not raw numbers) to measure and compare SSIs and other events monitored over time. IP uses appropriate methodology, numerators, and denominators for calculating rates.1,2 To ensure consistent surveillance data, the IP uses standardized data collection forms and CDC NHSN definitions and methodology.1 Facility Evidence of Compliance Recommended Practices Recommended Practices Examples of Compliance VII. Reporting and using surveillance information: A. There should be a plan for the distribution of surveillance information. B. Surveillance results should be reported to those who are most able to impact and improve patient care. C. Reporting should be done on a systematic, ongoing basis. Facility Evidence of Compliance A written Annual Surveillance Plan describes the persons and committees to whom surveillance reports are distributed. Surveillance findings are reported to the Quality Assurance/Performance Improvement Committee (QAPI) Reports on SSIs and compliance with safe injection and hand hygiene protocols are distributed quarterly to the Medical Director and QAPI. Essential Elements Essential Elements Examples of Compliance A written plan should serve as the foundation of the surveillance program. The ASC has a surveillance plan that is based on the populations(s) served and services provided. The plan includes: a brief description of the facility a description of the types of services provided and the population (s) served the objectives of the infection surveillance program a description of the surveillance indicators used the methodology for data collection (e.g., total or targeted; sources of information; use of a standardized data collection form) the methodology for calculating rates a description of the surveillance definitions used the types of surveillance reports, and how often and to whom they are distributed the process for evaluating the effectiveness of the program CDC NHSN definitions are routinely used.1 IP consistently uses NHSN methodology (including numerators and denominators specified by the CDC) for calculating SSIs.1 The IP is a healthcare professional who has taken a training course provided by APIC. The IP understands epidemiologic principles and Surveillance definitions and rate calculations are applied consistently over time. Personnel resources need to be appropriate for the type of surveillance being performed. Facility Evidence of Compliance Essential Elements Essential Elements Other essential resources, such as computer support, information and technology services, clerical services, and administrative understanding and support are available. The surveillance program (including surveillance processes and data), as part of the overall infection prevention and control program, should be evaluated at least annually. Evaluation methods may include qualitative assessments, but should also be based on quantitative changes (e.g., improvements or decline in rates). Examples of Compliance Facility Evidence of Compliance surveillance techniques. The IP has a personal computer linked to the ASC’s computer system and the Internet. The IP has access to clerical help. The Medical Director ensures that the IP has the necessary resources to manage the program in accordance with CMS, state and accrediting agency requirements. The ASC’s Annual QAPI Report outlines the findings of the annual evaluation of the IC Surveillance Program including the indicators measured and results of performance improvement activities related to infection prevention. Recommended practices and essential elements based on: Lee TB et al. Recommended Practices for Surveillance: Association for Professionals in Infection Control and Epidemiology. Am J Infect Control 2007;35:427-40 (available on APIC website) 1. 2. CDC National Healthcare Safety Network (NHSN) Manual: Patient Safety Component Protocols available at: http://www.cdc.gov/nhsn Lee T et al. Recommended Practices for Surveillance: APIC. Am J Infect Control 2007;35:427-40 (available on APIC website) Tool adapted from “Tool for Assessing a Surveillance Program” in Arias K. Surveillance Programs in Healthcare Settings. 2nd ed. Association for Professionals in Infection Control and Epidemiology, Inc: Washington, DC. 2009 Updated Feb 2014