Insertion and care of a Central Venous access device CVAD in adults
advertisement

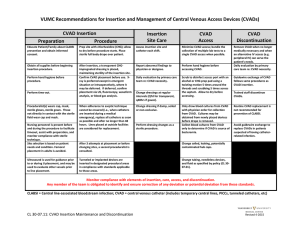
PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 TITLE Clinical Guidelines for insertion and immediate care of a Central Venous access device (CVAD) (adults ) MANAGER / COMMITTEE RESPONSIBLE Sean Elliott. Anaesthetics Consultant Barry Buchanan. IV Therapy Lead Nurse DATE ISSUED 13.01.2009 VERSION 1 REVIEW DATE January 2010 AUTHOR Sean Elliott. Anaesthetics Consultant Barry Buchanan. IV Therapy Lead Nurse Equality Impact Assessment has been completed Sean Elliott. Anaesthetics Consultant Barry Buchanan. IV Therapy Lead Nurse RATIFIED BY Professional Advisory Committee – 07.01.2009 AMENDMENTS RECORD DATE PAGE COMMENTS APPROVED BY CONTENTS: 1 2 3 4 5 6 7 8 9 INTRODUCTION STATUS PURPOSE SCOPE DEFINITIONS CLINICAL PRACTICE GUIDELINE SUPPORTING EVIDENCE DUTIES AND RESPONSIBILITIES TRAINING APPENDICES: APPENDIX I: AUDIT DOCUMENT 1 APPENDIX II: AUDIT DOCUMENT 2 APPENDIX III: Comment on NICE Technology Appraisal Guidance No. 49 on the use of Ultrasound Locating Devices for Placing Central Venous Catheters APPENDIX IV: CVC Insertion and Management Form Control Date: 05/02/16 Page 1 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 1. Introduction Insertion of a CVAD is a procedure which while useful carries some significant risks. This policy includes guidance from NICE and the CVC care bundle from the Institute for Healthcare Improvement to ensure that the procedure is done by the right person, in the correct manner, in the right place using the correct equipment. Subsequent care of the CVAD is described in another policy by Simon Freathy. 2. Status Clinical Guidelines for insertion and care of a Central Venous access device (CVAD) (adults) 3. Purpose CVAD’s are inserted to provide central venous access for either diagnostic or therapeutic purposes. Indications; a) Longer term intravenous fluid or drug therapy (usually for more than 3-4 days). b) Monitoring central venous pressure in seriously ill or physiologically unstable patients c) Parenteral nutrition and infusion of other substances that need diluting in the central circulation (chemotherapeutic agents, hyperosmolar solutions, inotropes and vasopressors etc). d) Specialised uses e.g. haemodialysis, plasmapheresis Complications include thrombosis, pain, local or systemic infection, catheter embolism, pneumothorax, haemorrhage, cardiac tamponade and dysrhythmias. These clinical guidelines outline how these risks to patients may be reduced. 4. Scope These guidelines apply to all health care professionals inserting and managing CVAD’s in the Trust. For Central Venous cannulation in paediatric areas, please refer to “Clinical Guidelines for Central Venous Cannulation (paediatrics)” For Peripherally-inserted Central Catheters (PICC lines) please refer to Peripherally-inserted Central Catheters (ADULT) This document does not refer to cardiac catheterisation or pacemaker insertion. 5. Definitions Central venous access device A vascular access device, the tip of which is placed in one of the great veins, for one of the above indications. Health care professional A registered or trained member of staff, including but not exclusively nurses, doctors and operating department practitioners. Control Date: 05/02/16 Page 2 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 6. Clinical Practice Guideline Action Identify patient and discuss with patient the need for a CVAD, obtaining consent for procedure, establishing whether patient has any known allergies or contraindications to CVAD insertion. The individual inserting the CVAD will have been appropriately trained in the landmark and/or ultrasound technique. Where possible, ultrasound imaging guidance should be used as the technique of choice for insertion of CVAD’s into the internal jugular vein in adults. Except in cases of emergency insertion, and according to clinical judgement, areas such as treatment rooms or operating theatres will be used for line insertion. The Trust CVAD (or CVC) pack will be used for the insertion of CVADs. In general, a line with the minimal number of lumens required should be used. Choose appropriate insertion site, weighing up risks and benefits. Consider that line infection rates seem to be lower with subclavian vein placement. Full barrier asepsis will be used for line placement, including surgical scrub technique, sterile gown and gloves. Skin will be prepared with 0.5% chlorhexidine in 70% alcohol unless the patient is allergic to this solution when iodine may be used. A Chest X-ray is required for subclavian and internal jugular lines. By employing an appropriate level of monitoring, ensure that the patients’ condition has not changed during the CVAD insertion procedure, in particular be vigilant for signs of pain, abnormal swelling, excessive bleeding, respiratory distress, cardiac arrhythmias, hypotension, syncope etc. On each line insertion, a Trust audit form will be completed as part of Daily care bundle form. These forms will form the clinical documentation for line care. (See attached form). The insertion procedure should be documented in the patient’s notes (including the date, time and location, the insertion site, use of ultrasound, the name and grade of the operator, details of the skin preparation and anaesthetic used the type of line and length inserted the lot number of the CVAD and the results of the CXR. Where possible patients with CVADs should be nursed in higher dependency areas. Patients with CVADs will be reviewed regularly by the Iv Therapy Team Nurses caring for patients with CVADs should be familiar with the appropriate policy. The “Daily checklist” for line care will be completed for each patient. Nursing staff are encouraged to challenge the need for a CVAD in a patient. The nurse will contact the Iv Therapy Team. Control Date: 05/02/16 Rationale To ensure patient is informed of procedure and that the risk of CVAD insertion is balanced against the clinical benefit. The operator will be familiar with the appropriate technique and potential complications of CVAD insertion. To be compliant with NICE guidance September 2002. To perform the procedure safely and in a sterile manner, demands adequate space, lighting and level of cleanliness. The pack has been designed to offer best value, ease of use and optimal sterility for the procedure. Unused lumens are associated with increased risk of line infection. Optimum site will vary dependant upon both patient factors and operator factors. To reduce risk of infection to patient, and risk of exposure of operator to blood-borne pathogens. To ensure correct placement and check for complications such as a pneumothorax Complications include thrombosis, pain, local or systemic infection, catheter embolism, pneumothorax, haemorrhage, cardiac tamponade and dysrhythmias Documentation will improve infection control surveillance, act as audit tool and trigger patient visits by the Iv Therapy Team. To provide evidence of the intervention for subsequent reference. The incidence of line sepsis increases as the intensity of nursing care reduces. The activity of such teams has been shown to reduce line infection rates. The incidence of line sepsis increases with the length of time it is in place. Page 3 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 7. Supporting evidence Centers for disease control and prevention (2002) Guidelines for the prevention of intravascular catheterrelated infections. Institute for Healthcare Improvement (2007) Central line care bundle (www.ihi.org) NICE (2002) Guidance on the use of ultrasound locating devices for placing central venous catheters Portsmouth Hospitals NHS Trust (2006) Advice for Medical Staff on Good Practice in Infection Control Maki D G et al (1991) Prospective randomised trial of povidone iodine, alcohol and chlorhexidine for prevention of infection associated with CVC and arterial catheters Lancet 338, 339 - 43 8. Duties and responsibilities Supervisors of clinical practice will be responsible for monitoring compliance with the guidelines on an ongoing basis. The Intravenous therapy Nurses will audit compliance as part of the infection control clinical practice audit process. A snapshot audit to monitor clinical practice during CVAD insertion and subsequent care will be undertaken annually. 9. Training Prior to undertaking any CVAD insertion procedure, staff must be able to demonstrate clinical competence and a clear understanding of the underlying principles of practice. This will be achieved by: Medical staff; PRHO’s and SHO’s should be trained by recognised Trust trainers on local guidelines and principles of practice. Specialist Registrars will be assumed competent unless identified otherwise by their supervisor. If problems are identified, the staff member will be required to complete a period of supervised clinical practice. Ultrasound guidance There are lead clinicians in each clinical area to provide training in the use of this technique. Individuals will have attended this training and/or attended an external course before using Ultrasound guidance for line insertion. Control Date: 05/02/16 Page 4 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 APPENDIX I: AUDIT DOCUMENT 1 The aim of this audit document is to establish levels of familiarity with the Portsmouth Hospitals Central Venous Cannulation policy and to assess compliance with said policy on a “snapshot” basis. The intention is to perform this audit snapshot annually and to use the results to guide future educational interventions. The standard that should be achieved is 100% compliance in all clinical areas. For each observed / performed CVAD insertion; Location / Ward area ……………………………………………………….………. Grade of Operator …………………………………………………………………. Indication for CVAD insertion ……………………………………………………… Type of CVAD .……………………………………………………………………… Explanation of procedure to patient Yes / No / N/A Surgical Scrub Yes / No Skin preparation solution ………………………………………………………….. Ultrasound localisation used yes / no Successful insertion of CVAD Yes / No Number of attempts ………………………………………………………………… Action taken if failed ……………………………………………………………….. Type of fixation ……………………………………………………………………… Appropriate sharps precautions demonstrated Yes / No Application of dressing Good / Bad Documentation in notes Yes / No Comments and agreed action: Completed by: Date Control Date: 05/02/16 Page 5 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 APPENDIX II: AUDIT DOCUMENT 2 The aim of this audit document is to establish levels of familiarity with the Portsmouth Hospitals Central Venous Cannulation policy and to assess compliance with said policy on a “snapshot” basis. The intention is to perform this audit snapshot annually and to use the results to guide future educational interventions. The standard that should be achieved is 100% compliance in all clinical areas. For each “in situ” CVAD in the clinical area; Location / Ward area ……………………………………………………….………. Indication for continuing CVAD …....……………………………………………… Type of CVAD ………………………………………………………………………. State of dressing Good / Bad Documentation in notes referring to CVAD in last 24hrs Yes / No Comments and agreed action: Completed by: Date Control Date: 05/02/16 Page 6 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 APPENDIX III: Comment on NICE Technology Appraisal Guidance No. 49 on the use of Ultrasound Locating Devices for Placing Central Venous Catheters There are a growing number of clinicians who believe that, given appropriate equipment and training, the use of 2D ultrasound represents a major advance in the safety of central venous cannulation. NICE reviewed the available literature and produced a guidance document in 2002. A number of medical bodies responded in the consultation period by raising concerns that a recommendation to always use 2D ultrasound would produce major problems as follows: 1. Subsequent failure to use 2D ultrasound could have major medico-legal implications. 2. In certain situations such as ITU or theatres, serious delays in instigating monitoring and subsequent treatment could occur. 3. It is relatively impractical in the emergency situation. 4. It would result in deskilling with regard to use of the landmark technique. 5. There would be a significant financial burden on the Trust, since a large number of machines would be required (20-30) and training would be necessary for all users. The NICE guidance acknowledges these concerns as follows; Two-dimensional (2-D) imaging ultrasound guidance is recommended as the preferred method for insertion of central venous catheters (CVC’s) into the internal jugular vein (IJV) in adults and children in elective situations. The use of two-dimensional (2-D) imaging ultrasound guidance should be considered in most clinical circumstances where CVC insertion is necessary either electively or in an emergency situation. 4.3.3 4.3.5 4.3.6 7.6 Given the constraints outlined in 4.2.2, the Committee concluded that there was evidence of both the clinical and cost effectiveness of 2-D imaging ultrasound guidance as an adjunct for placing CVC’s in the majority of clinical scenarios, but that the degree to which this technology would be most suitably applied would vary according to the clinical situation and the competence/previous experience of the operator. In addition, there could be potential benefits for patients arising from reduced discomfort from the procedure and reduced risk of complications compared with the landmark method, particularly for IJV insertions. While accepting that, from a patient’s perspective, 2-D ultrasound imaging guidance in CVC insertion might be more appropriate and probably superior to the traditionally used landmark method in many circumstances, the Committee also considered the financial and service implications of purchasing the required equipment and of training sufficient numbers of competent practitioners. The Committee also considered that although 2-D ultrasound imaging guidance in CVC placement may eventually become the routine method for placing CVC’s, the landmark method would remain important in some circumstances, such as emergency situations, when ultrasound equipment and/or expertise might not be immediately available. Consequently, the Committee thought it important that operators maintain their ability to use the landmark method and that the method continues to be taught alongside the 2-D-ultrasound-guided technique. Healthcare practitioners should consider the most appropriate method of CVC insertion that is in the best interest of the patient in his or her specific clinical situation, particularly in terms of minimising the risk of adverse events such as failed catheter placements or catheter placement complications. Trusts should recognise that the decision to use 2-D imaging ultrasound guidance or the landmark method will be informed by: Control Date: 05/02/16 Page 7 of 8 PORTSMOUTH HOSPITALS NHS TRUST Multi-Professional Guidelines Insertion and care of a Central Venous access device (CVAD) (adults) Issue 1. 13.01.2009 APPENDIX IV: CVC Insertion and Management Form IV team contacted (Bleep 1494) YES / NO (please delete as appropriate) Control Date: 05/02/16 Page 8 of 8




![Jiye Jin-2014[1].3.17](http://s2.studylib.net/store/data/005485437_1-38483f116d2f44a767f9ba4fa894c894-300x300.png)


