04_INTRODUCTION TO NEUROIMAGING
advertisement

Nervous system basic concepts
FUNDAMENTALS OF NEUROIMAGING TECHNIQUES
Dr. Bruce Giffin
Tuesday, April 3, 2007 11:00 AM
LEARNING OBJECTIVES:
1
Explain the advantages of the skull x-ray as a preliminary investigation in head injured patients.
2
Describe the principles of computerized tomography (CT) scanning, magnetic resonance imaging
(MRI), MR angiography (MRA), and MR venography (MRV)
3
List the advantages / disadvantages of MRI compared to CT scanning and the usefulness of
contrast agents.
4
Describe the tissue characteristics on CT scanning and MRI.
5
Be able to interpret the scans assigned in the self-study module.
6
List clinical uses of angiography, functional MRI (fMRI), single photon emission tomography
(SPECT) and positron emission tomography (PET).
After a complete history and physical examination, images of the skull, the brain
and its vessels, and spaces in the brain containing cerebrospinal fluid (CSF), can be a
tremendous aid in the localization of lesions. In this presentation we will overview the
fundamental principles of CNS imaging techniques and apply these techniques to the
visualization of basic brain anatomy. To help you develop skills at interpreting CT scans
and MR images, you will be assigned images in the Neuroimaging Lecture Image
Collection on Blackboard and Internet websites containing computerized tomography
(CT) scans and MRIs. We will use the standard convention for axial CT and MRI images
in which we will look at the cross-section as if we are at the foot of the patient’s bed. So,
the patient’s right side is on the left side of the image. Previously the views shown in
most images have been lateral, anteroposterior (frontal), or oblique.
However, since the introduction of CT which
displays sections in the horizontal (axial)
plane, magnetic resonance imaging, and
other methods it is possible to display
sections of the head in the sagittal and
coronal (frontal) planes.
NOTE: The images shown during this
presentation {Image #] can be viewed in the
NEUROIMAGING LECTURE IMAGE
COLLECTION which is available on Blackboard.
Read the section at the beginning of the
course syllabus entitled “Neuroimaging
Self-Study Module” for a description of how to access images.
SKULL X-RAY
Skull radiography with x-rays is an excellent means of imaging calcium and its
distribution in and around the brain especially when more precise methods are not
available. Learn to distinguish normal skull markings and sites of calcification (pineal
gland and choroid plexus). Look for the following:
[IMAGES #1,2]
FRACTURES
BONE HYPEROSTOSIS (hypertrophy of bone)
ABNORMAL CALCIFICATION-tumors, e.g. meningioma
MIDLINE SHIFT-if pineal is calcified
More specific views depend upon clinical conditions:
BASE OF SKULL-cranial nerve palsies
OPTIC FORAMINA-progressive blindness
SELLA TURCICA-visual field defects
PETROUS/INTERNAL AUDITORY MEATUS-sensorineural deafness
COMPUTED TOMOGRAPHY
Computed tomography (CT) or computed axial tomography (CAT) allows direct
imaging of the living brain and permits inspection of cross sections of the skull, brain,
ventricles, cisterns, large vessels, falx, and tentorium. A pencil beam of x-ray traverses
the patient’s head and a diametrically opposed detector measures the extent of its
absorption. Computer processing, multiple rotating beams and detectors arranged in a
complete circle around the patient’s head enable determination of absorption values for
multiple small blocks of tissue (voxels). Reconstruction of these areas on a twodimensional array (pixels) provides the characteristic CT scan appearance. For
routine scanning, slices are 5-10 mm wide. A series of 10-20 scans, each reconstructing
a slice of brain, is usually required for a complete study. An intravenous
iodinated water-soluble contrast medium is administered when the plain scan reveals an abnormality
or if specific clinical indications exist, e.g. suspected
arteriovenous malformation or intracerebral abscess
which may appear normal in the plain scan. Intravenous contrast shows an area with increased vascularity or with impairment of the blood-brain barrier.
Intrathecal water-soluble contrast medium combined with CT scanning outlines the basil cisterns,
the spinal cord and the lumbosacral nerve roots.
[IMAGES: #6,7,9,12,13,55,56,58,65,71,86]
Tissue Characteristics on CT
Air and fat have similar low attenuation and are readily identified
CSF is similar in attenuation to water
Gray and white matter are denser than CSF and are normally different enough to
be distinguished from each other
Flowing (normal) blood is denser than brain
Acute hematoma is denser than flowing blood due to clot retraction and loss of
water
Pathologic processes in the brain are often detected by CT because of the
presence of surrounding edema which is less dense than normal brain
Calcification is readily detected
Interpretation of the Cranial CT Scan
Before contrast enhancement notice:
(1) VENTRICULAR SYSTEM
DENSITY
(5) ABNORMAL TISSUE
Size
Identify the site, and whether the
lesion lies within or without the
brain substance
Position
.
Note the “MASS EFFECT”:
-midline shift
-ventricular compression
(2) WIDTH OF CORTICAL
SULCI AND THE
SYLVIAN FISSURES
High density
Blood
Calcification-tumor
Arteriovenous
-malformations
-aneurysms
(3) SKULL BASE AND VAULT
(Calcification of the pineal gland,
choroid plexus, basal ganglia and
falx may occur in normal scans)
Hyperostosis (hypertropy of bone)
Osteolytic lesion (bone resorption)
Depressed fracture
Low density
Infarction (arterial/venous)
Tumor
Abscess
Edema
(4) MULTIPLE LESIONS may result from:
Tumor -metastases
-lymphoma
Abscesses
Granuloma (chronic inflammatory lesion)
Infarction
Trauma
After contrast enhancement:
Vessels of the circle of Willis appear in the basal slices. Look at the extent
and pattern of contrast uptake in any abnormal region. Some lesions may only
appear after contrast enhancement.
MAGNETIC RESONANCE IMAGING (MRI)
[IMAGES: #14,18,19,21,24,28,29,30,31,60,64,70,72,75,77,80]
This imaging technique produces cross-sectional images that are similar to those
of CT, but the physical basis for MRI is completely different. MRI is based on the
physical phenomenon called nuclear magnetic resonance (NMR). Certain atomic
nuclei when placed in a uniform magnetic field and subjected to a radiofrequency (RF)
pulse will emit a pulse of radiofrequency in response. This ‘resonance’ can be measured
and contains information about the stimulated nuclei. To obtain an MR image the patient
is placed inside a powerful magnet. The body tissues contain atomic nuclei that act like
small spinning bar magnets. When placed in a strong magnetic field the nuclei respond
at resonance to produce an RF signal which is detected and stored. By varying the
magnetic field and the RF pulse the resultant data can be stored and processed to
produce images of the body tissues. The most commonly utilized nucleus is the
hydrogen (single proton) due to its strong RF signal and its natural abundance in
biological tissues. The image, a picture of the density or concentration of protons in body
tissues, is generated by computer processing of the received signal with reconstruction
similar to that used in CT. Image intensity varies with proton relaxation properties
(discussed below) and depends upon their chemical composition and binding. The MR
images can be acquired in any orientation, including axial, coronal, sagittal, and oblique
planes.
Here’s a brief, simplified outline of the process which will define some
terminology that should begin to become familiar to you:
The powerful magnetic field causes some of the nuclei to align the axis of their
spins along the vertical magnetic field-think of the proton in a low-energy state
(below)
The magnetization vector is then briefly rotated (usually through 90 or 180
degrees) by the application of a RF pulse
The tissue acquires energy during this RF pulse with the spinning nuclei now
aligned in the new horizontal field (excitation) and spinning synchronously or “in
phase” with one another-think of this as a high-energy state
This energy is released when the RF excitation is stopped and the protons are
free to return to the orientation of the vertical magnetic field (relaxation)
The emission of energy is in the form of a brief pulse of RF
This signal is detected and stored; its characteristics reflect the quantity and state
of the atoms in the tissue
The exciting RF pulse and the point in time at which the resonant signal is
detected and measured can be varied, and is known as the pulse sequence
Two signals are obtained from the proton’s realignment with the vertical magnetic field.
These are measured as time constants: T1 and T2. The tissue molecular structure and
chemistry affect the T1 and T2 signals. Small changes in tissue chemistry and
differences between normal and abnormal tissue can alter the values of T1 and T2
T1 RELAXATION TIME:
Also called “spin-lattice relaxation”
Reflects the loss of energy by nuclei
to their local environment
Rate of this relaxation is influenced
by nonexcited molecules in the
surrounding tissue
Time required for the protons to
return to their original alignment
within the vertical magnetic field
following the RF pulse
Time constant (time it takes 63% of
the vertical magnetization to recover
in the tissue)
Generally speaking: the greater the
proportion of free water present, the
longer the T1 relaxation time
T2 RELAXATION TIME:
Also called “spin-spin relaxation”
Dependent on nuclear spins going
out of phase with those surrounding
them (lose their phase coherence)
Occurs quickly and results largely
from the loss of energy to spinning
nuclei nearby
Time constant (time it takes for 63% of coherency to be lost)
This parameter is particularly sensitive for the detection of disease processes
(the type and status of health of tissue determine how much water is in its cells,
where it is located, and what is dissolved or suspended in it; that, in turn, has an
impact on the motions and local magnetic environments of individual water
molecules and will affect the relaxation time)
Another parameter, which has useful clinical application, is flow effects. When moving
blood is magnetized it has moved before the resonant signal can be received and leaves
a signal void at the appropriate site on the image. This phenomenon makes it possible
to identify vessels intracranially and elsewhere, and quantitative flow measurements are
possible as well.
These parameters, T1, T2, and flow effects, are currently the most important
components in the MR image. The images can be manipulated to give particular
emphasis to one or more of these parameters by an appropriate choice of pulse
sequence.
PULSE SEQUENCE
The pulse sequence is made up of one or more excitation RF (radiofrequency) pulses
followed (after a specific time interval) by a period during which the resonant signal
(MR signal) is received. After a further interval of time, the whole process is repeated.
The components of a pulse sequence are as follows:
Repetition time (TR): is measured in milliseconds and is the time from the
application of one RF pulse until the application of the next RF pulse. TR determines the
amount of relaxation (return of vertical magnetization) that is allowed to occur before the
next RF pulse is applied. This component of the pulse sequence determines how
much T1 relaxation has occurred.
Echo time (TE): is measured in milliseconds and is the time from the application of the
RF pulse to the peak of the resonant signal. The TE determines how much loss of spin
coherency is allowed to occur before the signal is read. This component of the pulse
sequence controls the amount of T2 relaxation that has occurred.
The application of RF pulses at certain repetition times and the receiving of signals at
pre-defined echo times produce the contrast in MR images.
Various image characteristics can be obtained by changing the times of sending and
receiving the radiofrequency pulse. Typically, relatively short TR and TE times are used
for T1-weighted images; long TR and TE times are used for T2-weighted images.
MRI IMAGE
PULSE TIMING
CNS STRUCTURE
APPEARANCE
T1-weighted
short TR/TE times
CSF
White matter
Gray matter
Fat
Black
Bright
Gray
Bright
T2-weighted
long TR/TE times
CSF
White matter
Gray matter
Fat
Bright
Dark
Gray
Dark
NOTE: MRI can demonstrate extraordinary anatomical detail by using reverse-contrast
T2-weighted images. In this procedure, which reverses the contrast of a T2-weighted
image, gray matter looks gray, white matter looks white, and CSF (in ventricles and
subarachnoid space) looks black. Bone, air and flowing blood look white.
Advantages of MRI (compared to CT scanning):
1.
2.
3.
4.
Best (highest resolution) images possible of brain and spinal cord
Can select any plane, e.g., coronal, sagittal, oblique
No ionizing radiation
Detects pathology often missed by CT (foci of demyelination, inflammation, early
metastases
5. Scan not affected by bony artifact
6. No allergic or toxic reactions to iodinated contrast agents
Disadvantages:
1.
2.
3.
4.
More sensitive to patient motion artifact
Claustrophobia (in closed models)
Cannot use if pacemaker or ferromagnetic implant is present
Slow scanning time prohibits monitoring of sick, medically unstable patients
MRI Characteristics of Some Common Structures
Fat appears very bright on T1-weighted images and appears dark on routine T2weighted images
Water appears relatively dark on T1-weighted images and very bright on T2weighted images
CSF and vitreous humor are very bright on T2-weighted images and dark on T1weighted images
Edema, which is present in many pathological processes in the brain and spinal
cord is bright on T2-weighted images
White matter is brighter than gray matter on T1-weighted images
Gray matter is brighter than white matter on T2-weighted images
Air and dense bone contain few mobile hydrogen protons and appear black on
MRI
Paramagnetic Enhancement
[IMAGES: #30,31]
Some substances, e.g., gadolinium, induce strong local magnetic fields - particularly
shortening the T1 component. After intravenous administration, leakage of gadolinium
through regions of damaged blood-brain-barrier produces marked enhancement of the
MRI signal, e.g., in ischemia, infection, tumors, and demyelination. Gadolinium may also
help differentiate tumor tissue from surrounding edema.
Interpretation of Abnormal MRIs
[IMAGE: #57]
Look for structural abnormalities and abnormal intensities indicating a change in tissue
T1- or T2-weighting in relation to normal gray and white matter. (A prolonged T1
relaxation time gives hypointensity, i.e., more black; a prolonged T2 relaxation time
give hyperintensity, i.e., more white.)
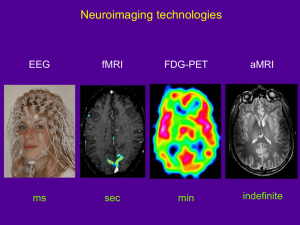
Functional MRI (fMRI) [IMAGE: #50]
Functional MRI locates neural activity by examining regional blood flow in the
brain. In the region of neuronal activity the supply of oxygenated blood is greater than
its consumption (the body actually overcompensates for the increased metabolic
activity), leading to a higher than normal ratio of oxygenated to deoxygenated blood.
Because the two forms of hemoglobin have different effects on the dephasing of protons,
the produce different magnetic resonance signals.
ANGIOGRAPHY
MRA Angiography (MRA) [IMAGE: #36,39,40, 41,42]
Rapidly flowing protons can create different intensities from stationary protons
and the resultant signals obtained by special sequences can demonstrate vessels,
aneurysms and arteriovenous malformations. Vessels displayed simultaneously may
make interpretation difficult, but selection of a specific MR section can demonstrate a
single vessel or bifurcation. By selecting a specific flow velocity, MRA will show either
arteries or veins (magnetic resonance venography-MRV).
Cerebral Angiography [IMAGE: #35]
A neurodiagnostic procedure in which a vessel abnormality such as occlusion,
malformation, or aneurysm is suspected. Angiography can also be used to determine
whether the position of the vessels in relation to intracranial structures is normal or
pathologically changed. Intra-arterial injection of contrast remains the standard
angiography technique, either imaged directly on x-ray film or by digital subtraction (see
below). Arteriovenous fistulas or vascular malformations can be treated by interventional
angiography using balloons, a quickly coagulating solution that acts as a glue, or small,
inert pellets and coils that act like emboli.
Digital Subtraction Angiography (DSA) [IMAGES: #37,38]
A modern form of angiography, digital subtraction angiography is a technique in
which extraneous tissue in the image is erased, or subtracted. DSA depends upon high
speed digital computing. Exposures taken before and after the administration of contrast
agents are instantly subtracted ‘pixel by pixel’. Data manipulation allows enhancement of
small differences in shading as well as magnification of specific areas of study. DSA
results in improved contrast sensitivity, permitting the use of much lower concentrations
of contrast material.
Complications of angiography
The development of non-ionic contrast medium has considerably reduced the
risk of complication during or following angiography. There is a risk of cerebral ischemia
caused by emboli from an arteriosclerotic plaque broken off by the catheter tip,
hypotension, or vessel spasm following the contrast injection. The reduced amount of
contrast needed for DSA carries less risk. In some cases a mild sensitivity to the
contrast occasionally develops, but this rarely causes severe problems.
RADIONUCLIDE IMAGING
Positron Emission Tomography (PET) [IMAGES: #43,44,45]
PET is a sensitive method of imaging based on the detection of trace amounts of
radioactive isotopes. These isotopes are used to tag biological molecules of interest by
emitting positrons. The tagged tracers are injected into the bloodstream (or inhaled) and
after reaching the brain permit the imaging of regional changes in blood flow and
alterations in the metabolism of glucose in various regions of the brain. These
parameters are indicative of changes in neural activity. Neurotransmitters labeled with
radioisotopes permit the imaging of the binding and uptake of specific neurotransmitters.
Multiple pairs of detectors and computer processing techniques enable quantitative
determination of local radioactivity for each voxel within the imaged field. Reconstruction
using similar imaging techniques to CT scanning produces the positron emission scan.
Single Photon Emission Tomography (SPECT) [IMAGES: #46,47,48,49]
SPECT makes use of radioisotopes that emit single photon radiation, usually gamma
rays, but unlike conventional scanning, acquires data from multiple sites around the
head. Similar computing to CT scanning provides a two-dimensional image depicting
the radioactivity emitted from each ‘pixel’. This gives improved definition and localization.
Various ligands have been developed but a technetium labeled derivative of propylamine
oxime (MHPAO) is the most frequently used. This tracer represents cerebral blood flow
since it rapidly diffuses across the blood-brain-barrier, becomes trapped within the cells,
and remains long enough to allow time for scanning.
Clinical and Research Uses
PET scanning is of particular value in elucidating relationships between cerebral
blood flow, oxygen utilization and extraction in focal areas of ischemia and infarction. It
has also been used to study patients with dementia, epilepsy, and brain tumors.
Identification of neurotransmitter and drug receptor sites has aided in the understanding
and management of psychiatric and movement disorders. Functional MRI promises to
yield maps of the brain that can be related to specific behavioral events.
SELECTED REFERENCES
Bushong, S. (1996) Magnetic Resonance Imaging: Physical and Biological Principles.
Moseby: St. Louis. MO.
Cordoza, J. and Herfkens, R. (1994) MRI Survival Guide. Lippincott Raven: New York,
NY.
Dowsett, D.J., Kenny, P.A. and Johnston, R.E. (1998) The Physics of Diagnostic
Imaging. Chapman and Hall: London.
Hendee, W.R. and Ritenour, E.R. (1992) Medical Imaging Physics, 3rd ed. Mosby Year
Book: St. Louis, MO.
Kandell, E.R., Schwartz, J.H. and Jessell, T.M., eds. (2000) Principles of Neuroscience,
4th ed. McGraw-Hill:New York, NY.
Ness Aiver, M. (1996) All You Really Need to Know about MRI Physics. University of
Maryland: Baltimore.
Webster, J.G., ed. (1988) Encyclopedia of Medical Devices and Instrumentation. John
Wiley and Sons: New York, NY
Wolbarst, A.B. (1993) Physics for Radiology. Appleton and Lange: Norwark, CONN.
Wolbarst, A.B. (1999) Looking Within: How X-Ray, CT, MRI, Ultrasound and Other
Medical Images Are Created and How They Help Physicians Save Lives. University of
California Press: Berkley, CA.
NEUROIMAGING SLIDE COLLECTION INDEX
• The following is an index of all the slides that are currently in
the “Neuroimaging Image Collection” which is available for
your use through Blackboard. Many of these images are
referenced throughout this handout on “Introduction to
Neuroimaging”.
1 Normal skull x-ray image
2 Radiograph of the skull antero-posterior view
3 Dorsal view of the cerebral hemispheres - MRI (inverted
inversion recovery) and a CT
4 Axial CT scan of lateral ventricles with choroid plexus
5 CT image - contrast enhancement - horizontal section at the
level of the thalamus
6 horizontal (axial) CT scan
7 Axial CT of petrous at level of internal auditory canal
8 A horizontal (axial) CT scan
9 Iodinated contrast agent injected intravenously prior to the
horizontal CT scan
10 Computed tomography. (A) Infarction in the left middle
cerebral artery distribution. (B) Area of hemorrhage in the right
occipital lobe. (C) Blood in the basilar cisterns. (D) Large,
predominantly left frontal arteriovenous malformation in a
contrast-enhanced scan
11 Contrast-enhanced scan
12 Normal CT - Glioma revealed by CT
13 (A) Axial CT, middle cerebral artery stroke. (B) Similar section,
reperfusion. (C) Following contrast injection
14 Normal MRI images (T1/T2 weighting in relation to normal
grey/white matter)
15 (A) T1 weighted. (B) T2 weighted MRI. (C) Proton density
weighted MRI - sagittal sections.
16 Sagittal T1 section NWR image of the normal brain.
17 A parasagittal MR image
18 T1 weighted image - coronal section
19 T2-weighted image - coronal section
20 Examples of MR images (T2
weighted). (B) coronal section. (C) horizontal section.
21 T1-weighted and T2-weighted MR images - horizontal section
22 Sagittal MR image of cerebral lobes
23 Sagittal MR scan of ventricular system
24 Coronal MR scan of orbit with rectus muscle group
25 Coronal MR scan of flax cerebri and tentorium cerebelli
26 Axial MR scan through the hippocampus
27 Proton density image - coronal section
28 A proton density MRI image in a horizontal plane through the
level of insula
29 (A) This T2-weighted image forms the basis for the REVERSE
CONTRAST MR image - coronal section. (B) REVERSE
CONTRAST
30 Gadolinium enhancement. The left panel of each pair of
images is the unenhanced MRI. Images on the right were made
following injection of gadolinium. Upper left. T1 weighted (TR10002/TE-137). Lower left. Proton density-weighted (TR-2000/TE20). Right. Both T1-weighted (top, TR-650/TE-30; bottom TR500/TE-16).
31 Thoracic spine MRI. (A) Sagittal T2W1 reveals a large
intramedullary mass. (B) After gadolinium administration (T1W1)
32 (E) Axial CT through the left of the fourth ventricle and pons.
(F) Axial MRI (T1W1) through the level of the fourth ventricle and
pons.
33 (C) Axial CT though the level of the midbrain. (D) Axial MRI
(T1W1) through the level of the midbrain.
34 (A) Axial CT though the level of the internal capsule and basal
ganglia. (B) Axial MRI, T1-weighted image (T1W1), through the
level of the internal capsule and basal ganglia.
35 The major cerebral arteries seen by carotid angiography,
lateral projection
36 Magnetic resonance angiography showing the major
intracranial and extracranial arteries and veins. (A)
Anteroposterior view (B) Lateral view
37 Digital subtraction arteriogram. Lateral projection of the left
common cartoid bifurcation shows segmental stenosis of the
proximal internal cartoid artery.
38 Digital subtraction angiogram of the neck vessels, oblique
anterior view
39 Magnetic resonance angiography (MRA) of the
vertebrobasilar system
40 Magnetic resonance angiogram (MRA), demonstrating most
of the arterial supply of the brain
41 Magnetic resonance venography (MRV) primarily
demonstrating veins and venous sinuses.
42 Magnetic resonance venography (MRV) - anterior-posterior
view
43 PET scans reveal regions of the
brain involved in processing of visual information.
44 PET scan, labeled with 18-F(F-DOPA) to identify
dopaminergic fetal mesencephalic cells implanted in the
putamen of a patient with Parkinson’s disease.
45 PET scan of a horizontal section at the level of the lateral
ventricles.
46 Thrombolysis after middle cerebral artery (MCA) occlusion SPECT
47 SPECT image of a horizontal section through the head at the
level of the temporal lobe.
48 SPECT - Normal scan - detection of early ischemia in
occlusive or hemorrhagic cerebrovascular disease assessment of blood flow changes in dementia
49 SPECT- Evaluation of patients with intractable epilepsy of
temporal lobe origin
50 fMRI - The blood oxygen level detection (BOLD) signal is
superimposed on a transverse slice of the brain imaged by
anatomical MRI through the basal ganglia and thalamus
51 A single CT image shows an area of hemorrhage along the
falx in an elderly patient with amyloid angiopathy.
52 MR scan shows bilateral small, chronic subdural hematomas.
53 CT image of a horizontal section through the head of a 7-yearold child with noncommunicating hydrocephalus
54 Multiple sclerosis demonstrated by MRI
55 CT scan. (A) An arteriovenous malformation. (B) Several
metastatic tumors near junctions between grey and white
matter.
56 CT scan. (C) An epidural hematoma resulting from a skull
fracture and torn meningeal artery. (D) The same patient shown
in C, but with CT parameters set to show bone detail.
57 Isodense subdural hematoma on CT, and MRI appearance.
58 Hydrocephalus demonstrated by CT
59 Isodense subdural hematoma with minimal mass effect.
Axial, nonenhanced CT scan.
60 Multiple sclerosis (MS) CT versus MRI
61 A comparison of normal and hydrocephalic brains in a a
sagittal plane as seen in MRI.
62 Comparison of normal and hydrocephalic brains in the axial
plane as seen in MRI.
63 Comparison of normal and hydrocephalic brains in the
coronal place as seen in MRI.
64 MRI of a midsagittal section through the head showing
venous channels.
65 CT image showing an infarct caused by middle cerebral
artery occlusion.
66 MRI of a coronal section of the head showing an infarct
(arrows).
67 CT image of a horizontal section of the head, showing an
infarct caused
by a right-sided anterior cerebral artery occlusion.
68 CT image of a horizontal section through the head showing a
hematoma in the putamen.
69 CT image of a horizontal section through the head, showing
high densities, representing a subarachnoid hemorrhage in the
sulci.
70 MR image of a horizontal section through the head,
demonstrating an arteriovenous malformation.
71 CT image of a horizontal section through the head, showing a
right subdural hematoma causing a shift away from the lesion.
72 MR image of a horizontal section through the head, showing
a left subdural hematoma causing a midline shift.
73 CT image of a horizontal section through the head, showing
an extradural hematoma and intracerebral contrecoup lesion.
74 MRI of a horizontal section through the head at the level of
the lower pons and internal auditory meatus. A left acoustic
schwannoma with its high intensity is shown in the left
cerebellopontine angle
75 MRI of a parasagittal section through the lumbar spine with a
root tumor.
76 MRI of a sagittal section through the lower lumbar space.
Note the herniation of the nucleus pulposus at L4-5
compressing the cauda equina.
77 MRI of a horizontal section showing the lesions of multiple
sclerosis
78 MRI through the base of the brain in a patient with a pituitary
adenoma
79 CT image of a horizontal section through the head at the level
of the lateral ventricles in a patient with glioblastoma
multiforma.
80 MRI of a horizontal section - bilateral subdural hematoma
81 CT image, with contrast enhancement - meningioma.
82 CT images. Left: hydrocephalus. Right: brain tumor.
83 CT images. Left: brain tumor. Right: cerebral hemiatrophy.
84 CT images. Left: cerebral hemorrhage. Right: Traumatic
intracerebral hemorrhage.
85 CT scans. (A) Infarction in the left middle cerebral artery
distribution. (B) Area of hemorrhage in the right occipital lobe.
(C) Blood in the basilar cisterns. (D) Large, predominantly left
frontal arteriovenous malformation in a contrast-enhances scan.
86 CT scan at a level showing the lateral ventricles.
87 Hydrocephalus demonstrated by CT.
88 Glioma revealed in this proton density-weighted MRI.