Boiler-Plate Templates for Molecular Imaging Mice/Rat Procotols
advertisement

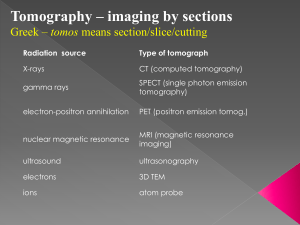
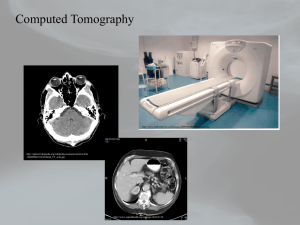
The Craniofacial Center’s Small Animal Tomographic Analysis (SANTA) Facility at the University of Washington School of Medicine May 2009 Boiler-Plate Language for Animal Protocols Please copy (or modify as required) the following information into your animal protocol or animal protocol amendment. Please only use the information that is appropriate to the species that you are using. If you have any questions or comments, please contact A/Prof Timothy Cox (phone: 206-616-9703; email: tccox@u.washington.edu) Introductory Paragraph for your Amendment: The Craniofacial Center’s Small ANimal Tomographic Analysis (SANTA) Facility provides state-of-the-art imaging of small animals and tissues to CHRMC and UW researchers and their collaborators. The facility is housed within the Department of Pediatrics, University of Washington School of Medicine, and currently offers in vivo micro-computed tomography (microCT) and optical projection tomography (OPT) imaging modalities. The former is suitable for ex vivo studies on a wide variety of small animals or tissues or in vivo studies on appropriately anesthetized mice, rats, and other small animals. The latter imaging modality is ideal for soft tissue and embryonic studies, including morphology, tissue composition, and 3D gene and protein expression profiles. The facility also provides high-end workstations for the analysis of collected data in both two- and three-dimensions. Description of Imaging Modalities: Tomographic imaging is a non-invasive technique that involves the collection of defined-thickness planar sections of an intact object. Individual sections can be analyzed or ‘reconstructed’ by computer algorithms into realistic 2D and 3D images of the scanned object that permits ‘visualization’ of internal structural detail. 1) Micro-Computed Tomography: Computed tomography is a procedure that is routinely performed on humans. Micro-Computed Tomography can be performed on intact tissue, deceased whole animals or on anesthetized animals and only differs from the technique used on humans by the fact that the resolution is ~100-1000x greater (ie. microns versus millimeters). The available Skyscan 1076 microCT is a low dose in-vivo X-ray scanner for slice imaging and 3D image reconstruction from small animals (up to 68mm wide and 200mm long) at isotropic resolutions of 9, 18 or 35 microns. 2) Optical Projection Tomography: OPT microscopy is a new technique that allows the 3D imaging of biological specimens up to 1.5 cm across at resolutions of as high as 3.1 microns. It was initially developed with the hope of enabling accurate measurement of 3D shapes. However, it has proven to be a fast method for determining the 3D distributions of gene expression patterns, labeled antibodies and cell-specific stains during embryo development or in isolated adult organs from small animals. OPT is based on the same principles as micro-computed tomography except that it employs either white, infra-red or UV light instead of X-rays. Examples of methods of sedation of small animals Animals will be anesthetized in accordance with the Principal Investigator’s approved protocol; the table below lists possible methods. TYPE OF ANESTHESIA SIDE EFFECTS Ketamine/ Xylazine Isoflurane Used for anesthesia. No side effects anticipated at the dosage used. Used for inhalation anesthesia and titrated to effect. No side effects anticipated at the dosage used. -1- The Craniofacial Center’s Small Animal Tomographic Analysis (SANTA) Facility at the University of Washington School of Medicine May 2009 Anesthesia Doses: DRUG/SPECIES MOUSE Ketamine / Xylazine 100 mg/kg Ket. / 10 mg/kg Xyl. given intraperitoneally; re-dose ketamine only at ½ dose Isoflurane 1-3% RAT 40-60 mg/kg Ket. / 3-5 mg/kg Xyl. mixed together and given intraperitoneally; re-dose ketamine only at 1/3 dose 1-3% Disposition of Animals Following Study: Any animal euthanized prior to scanning (ex vivo) or after completion of the scan (in vivo) will be conducted according to the Principal Investigator’s approved protocol. (Note: All protocols must include a euthanasia plan for each animal species if needed. In time, personnel within the facility will become available to assist with such procedures, however, this must be arranged with the Facility Director and will require addition of the person to the PI’s approved protocol.) Skyscan 1076 MicroCT specifications: X-Ray Source X-ray Source Energy Range X-ray Filtering X-ray Exposure to Animal 10W, <5um spot size, >10000h expected lifetime, air cooled sealed type Continuously variable peak energy 20-100kV 4-position automatic filter changer for energy filtration (no filter, Ti 0.025mm, Al 0.5mm, Al 1.0mm >99.9%purity) 0.1-0.5 Gy per scan typical X-Ray Detector CCD Number of Pixels Detectability 12bit cooled digital X-ray camera with fiber-optic coupling to scintillator 10 Megapixel (4000x2300) 9-14um Maximum Object Size for Reconstruction Single Scan Multi- Scan Mode ~25 mm single scan length x 68mm diameter 210mm length x 68mm diameter (~25mm steps) Scanning System Source-Detector Pair Physiological Monitoring Subsystem Miniature CMOS-camera with LED illumination Real-time local body movement detection Air / gas flow sensor Temperature sensor Chamber heating ECG-amplifier with "x-ray invisible" connections to the animal's body Software package Source-detector pair rotation with 0.02 deg. min. step size, 50um object positioning accuracy with 400mm travel, 50mm camera positioning/alignment with 1um accuracy, <10 microns overall stability during scanning Real time visualization of anesthetized animal during scanning Enables non-contact scanning synchronization with breathing Facilitates scanning synchronization with breathing Enables animal temperature measurement Occurs by airflow on the object bed For cardiac monitoring during scan ‘Runs’ animal physiological monitoring (temperature measurement and stabilization, body movement detection, pulse rate measurement in breathing and heart activitiy, etc.) and supports scanning synchronization with local body movements / breathing / heart rate. -2- The Craniofacial Center’s Small Animal Tomographic Analysis (SANTA) Facility at the University of Washington School of Medicine May 2009 Reconstruction Array 1. 2. 3. Format Pixel Size 9um 18um 35um 1000x1000 pixel to 8000x8000 pixel cross-section format Bioptonics 3001M OPT specifications: Physical characteristics: Compact desk-top scanner controlled directly from PC without using any additional interface cards Size: 925W x 270H x 300D Weight: 24kg Power: 100-240V AC, 50-60Hz, 300VA Computer connections: Scanner control: RS232 port Image output: IEEE1394 (FireWire) Magnification range: Field of view: 4x4 mm….20x20mm Illumination system: Automatic filter changer with independent intensity control for transmission light with following illumination options: White (transmission) 8 x white LEDs InfraRed (transmission) 8 x IR LEDs Fluorescence Metal-halide 120W UV Source with liquid light guide: › GFP – exciter 425nm/40 nm / emitter LP475 nm › GFP+ – exciter 480nm/20 nm / emitter LP515 nm › TXR – exciter 560/40 nm / emitter LP610nm Camera: CCD-Camera with high quantum efficiency sensor operated in on-chip integration mode: Resolution: 1024x1024 or 512x512 square pixels Dynamic range: >63dB (>1:1500) Exposure range: 1 ms - 10sec with 1ms step Image output: 12-bit digital with IEEE1394 (FireWire) interface Quantum efficiency: >70% Motorization & object manipulator: 6-axis micro-step controller with independent microprocessor control for each motor: Motorized zoom Motorized focus Motorized filter changer Object rotation: 0.9 degree full step, 0.1125 degree micro-step Motorized object alignment system Motorized object positioning with 50 mm travel -3-