Lecture 13
advertisement

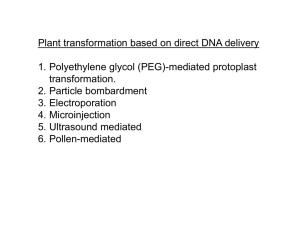
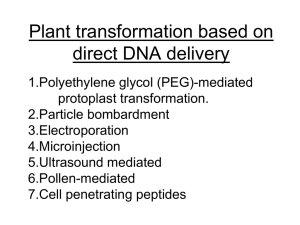
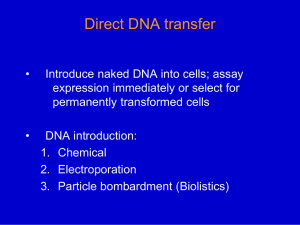
PLANT BIOTECHNOLOGY & GENETICS (3 CREDIT HOURS) LECTURE 13 INCORPORATION OF TRANSGENES INTO CROPS, METHODOLOGY OF PRODUCING TRANGENIC CROPS, CONFIRMATION OF PUTATIVE TRANSGENIC PLANTS & TRANSFORMATION EFFICIENCY POLYMERASE CHAIN REACTION SOUTHERN BLOTTING, NORTHERN BLOTTING, WESTERN BLOTTING, FUNCTIONAL ASSAY, PROGENY TEST, CROP SPECIES AMENABLE TO TRANSFORMATION INCORPORATION OF TRANSGENES INTO CROPS • Most of the methods currently used for plant transformation employ a technique for delivering the DNA into the cell without regard to its ultimate intracellular location. Once inside the appropriate cellular location by a chance process, the DNA is integrated into the chromosomal (or organellar) DNA, usually by a nonspecific recombination process. The exception is Agrobacterium-mediated transformation, which delivers the DNA specifically into the nuclear compartment with high efficiency and also provides a mechanism for its integration. • In plant transformation, two physical barriers prevent the entry of DNA into a nucleus, namely the cell wall and the plasma membrane. Most plant transformation methods overcome these barriers using physical and/or chemical approaches. In protoplast-mediated transformation, the cell wall is digested with a mixture of enzymes which attack cell wall components, yielding individual protoplasts that can be maintained intact using appropriate osmoticum in the medium. Entry of DNA is then facilitated by the addition of permeabilizing agents, such as polyethylene glycol, that allow DNA uptake, presumably by coating the negatively charged DNA inside the cell and various cellular compartments in a random process. INCORPORATION OF TRANSGENES INTO CROPS • A second method, often referred to as biolistic transformation, utilizes fine metal particles (typically tungsten or gold) coated with DNA that are usually accelerated with helium gas under pressure. Other methods, such as microinjection, sonication, and electroporation cause transient microwounds in the cell wall and the plasma membrane, allowing the DNA in the medium to enter the cytoplasm before repair or fusion of the damaged cellular structures. However, many of these methods are tedious and result in variable transformation efficiencies. • In all transformation techniques, the desired transgene is placed under the control of a promoter which produces high-level constitutive or inducible expression of the gene in specific or all tissues. In addition, another gene that allows detection or selection of the transformed cells is introduced in the same or different vector DNA. The presence of a screenable or selectable marker gene greatly simplifies the identification of transformed plants and increases the efficiency of recovery of transgenic plants. Typical screenable markers are the gus gene, encoding a β-glucuronidase, and the gfp gene encoding a green fluorescent protein. Selectable markers that have been most useful are those conferring resistance to antibiotics such as kanamycin, paromomycin, and hygromycin, or to herbicides such as bialaphos, glyphosate, or cyanamide. METHODOLOGY OF PRODUCING TRANSGENIC PLANTS • Most of the important crop species have been successfully transformed, at least in the laboratory. Two major approaches have been widely used to produce transgenic crop plants, both monocots and dicots. One is biolistic bombardment and the other is Agrobacterium-mediated transformation. In the biolistic protocol, the primary delivering system is the helium-powered gun. The transgene and the selectable marker are inserted in the vector between two unique sequences, called the left border and the right border, which are utilized in insertion of the T-DNA into the host chromosomal DNA. • Parameters involved in the biolistic gun include pressure (ranging from 900 to 1300 psi), particle size (0.6 to 1.1 µm), and type of material (gold and tungsten), target distance (7.5 to 10 cm), and target material (cell suspension, callus, meristem, protoplast, immature embryo). These parameters vary somewhat with the crop involved. Disadvantages of using the biolistic gun are low success frequency, high copy numbers that often are correlated with gene silencing, patent issues and cost. METHODOLOGY OF PRODUCING TRANSGENIC PLANTS Agrobacterium-mediated transformation may correct some of the weaknesses encountered with the biolistic approach and has been successful in rice, maize, sorghum, and other crops; its effectiveness with other crops, especially wheat, remains questionable. Further, some cultivar versus Agrobacterium strain specificity may limit the range of cultivars that can be successfully transformed. Development of reliable and efficient protocols are needed to improve the efficiency and range of both approaches in transforming crop plants. METHODOLOGY OF PRODUCING TRANSGENIC PLANTS Transformation techniques not involving tissue culture are desirable for crop species in which many cultivars do not respond to tissue culture. These techniques include soaking and vacuum infiltration transformation of Arabidopsis inflorescence with Agrobacterium, transformation via the pollen tube pathway and pollen transformation via biolistics. The dipping method adopted in Arabidopsis must be modified for cereal crops by altering the plant stage of infiltration, the concentration of bacterial culture, and the duration of treatment. METHODOLOGY OF PRODUCING TRANSGENIC PLANTS Further, each tiller (for wheat, barley etc) of the same plant must be kept separate. If this technique works, selection for transformants is made directly from seed-derived seedlings and tissue culture is avoided. Thus, genotypes that respond negatively to callus induction and plant regeneration will not present a problem; however, it is likely some genotypes will be more amenable to infiltration transformation than others. CONFIRMATION OF PUTATIVE TRANSGENIC PLANTS & TRANSFORMATION EFFICIENCY Commonly used methods to confirm the putative transgenic plants are polymerase chain reaction, Southern blotting, Western blotting, Northern blotting, functional assay (testing the presence of selectable marker and the target gene), in situ hybridization, and progeny analysis (segregation of the target gene). Not all transgenic plants produce the same amount of protein from the target gene and selection based on the Western blot is necessary. This is because a positive correlation usually exists between the effectiveness of the gene in the bioassay and the amount of protein it produces. For example, the level of rice chitinase accumulating in transgenic sorghum plants with the chi11 gene was positively correlated with resistance to sorghum stalk rot. POLYMERASE CHAIN REACTION PCR, a simple and rapid procedure, is utilized to confirm whether a putatively transgenic plant that has survived selection is indeed transgenic. Usually, two primers (one forward and one reverse) specific for the selectable marker (bar gene, for example) are used in a PCR reaction with genomic DNA extracted from the transgenic plants. A thermostable DNA polymerase amplifies the region between the two primers during the multiple amplification cycles of the PCR, which yields a DNA fragment of predicted size (the length equal to the number of base pairs between the two primers in the transgene). This fragment is easily detected on an agarose gel by staining with ethidium bromide. PCR is a very sensitive and rapid method for identification of transgenic plants in the seedling stage and requires only a small amount of plant tissue. SOUTHERN BLOTTING/ HYBRIDIZATION ANALYSIS In Southern blotting, DNA fragments from transgenic plants generated by digestion with restriction enzyme(s) are first separated according to fragment size by electrophoresis through an agarose gel. The DNA fragments then are transferred to a solid support, such as a nylon membrane or a nitrocellulose sheet. The transfer is affected by simple capillary action, sometimes assisted by suction or electric current. The DNA binds to the solid support, usually because the support has been treated to carry a net positive charge, or some other means of binding such as inducing covalent binding of the DNA to the support. DNA fragments maintain their original positions in the gel after transfer to the membrane. Hence, larger fragments will be localized toward the top of the membrane and smaller fragments toward the bottom. The positions of specific fragments can then be determined by “probing” the membrane. The probe consists of the DNA fragments of interest, such as a cloned gene, which has been labeled with a radioactive isotope or some other compound that allows its visual detection. Under the proper set of conditions, the denatured single-stranded probe will hybridize to its complementary single strands of genomic DNA affixed to the membrane. SOUTHERN BLOTTING/ HYBRIDIZATION ANALYSIS In this way, the size of the fragment on which the probe resides in the genomic DNA can be determined. In transgenic plant experiments, the Southern blot often is used to determine whether an introduced gene is indeed present in the plant DNA and whether multiple transgenic plants carry the introduced gene on the same size of DNA fragment (suggesting independent transformation events). The results of Southern blots also indicate whether a single copy of the gene has been inserted or if multiple copies are likely to be present. NORTHERN BLOTTING Northern blotting – the name was derived as a play on words from the Southern blot – is very similar to the Southern blot, except that instead of restriction enzyme-digested DNA, native RNA is separated according to size by electrophoresis through an agarose gel and then transferred to a solid support. The rest of the Northern blot procedure is very similar to that of the Southern blot and it is used to determine whether the introduced gene has been transcribed into messenger RNA and accumulates in the transgenic plant. WESTERN BLOTTING The Western blotting procedure detects the protein of the transgene in an extract of proteins prepared from various parts of the transgenic plants and is, therefore, an assay for a functional transgene. In this technique the proteins are first electrophoresed in an SDSpolyacrylamide gel and the proteins are then transferred to nitrocellulose membrane by electrophoretic transfer. The membrane is then treated with an antibody specific for the protein encoded by the transgene followed by a second antibody coupled to an enzyme, which can act on a chromogenic (or fluorogenic) substrate leading to visualization of the transgene protein with increased sensitivity. The expression level of the protein can be quantified using known amounts of the transgenic-encoded protein. FUNCTIONAL ASSAY When the selectable markers used are antibiotic-resistant or herbicideresistant genes, a functional assay can be made by spraying antibiotics or smearing herbicide on the leaves of those putative transgenic seedlings or plants in later segregating populations. Sensitive plants typically will turn brown and shrivel up whereas resistant (transgenic) plants will stay healthy and green. Such an assay provides the initial screening of large number of putative transgenic plants and reduces the work load by eliminating escapes during selection. PROGENY TEST With stable transformed genes, progeny testing should show the presence and activity of the selectable marker and target genes, such as the gene gfp encoding green fluorescent protein, or bar and disease resistance. However, segregation does not always follow the typical Mendelian fashion. For example, among the progeny in the Agrobacterium-mediated wheat transformation experiments reported, segregation in the T1 generation had ratios of 32:0, 1:34, 0:40 and 74:0 in addition to the expected 1:1, 3:1 or 15:1 ratios. This variability indicates aberrant segregation. However, in other cases, segregation follows the normal Mendelian pattern. For example, among six sorghum T0 transgenic plants produced by biolistic bombardment, all showed typical 3:1 segregation ratios in the T1 generation. CROP SPECIES AMENABLE TO TRANFORMATION • Any crop that is able to produce calli from explants and is capable of callus regeneration into plants with high efficiency is amenable to transformation using biolistic bombardment and Agrobacterium tumefaciens. However, it is known that response to tissue culture is highly genotype dependent. Furthermore, somaclonal variation; spontaneous genetic variations occurring in cells growing in vitro could occur during tissue culture processes. Thus, to confirm that the improved phenotype of the transgenic plants is due to the transgene, two controls should be included – one from seed-derived plants and the other from non-transformed tissue culturederived plants. • For those crops of genotypes that show a negative response to tissue culture, a transformation procedure independent from tissue culture should be considered and tested. It will be a great accomplishment to perfect a procedure bypassing tissue culture, because many cultivars of wheat and rice and inbred lines of maize and sorghum do not respond to tissue culture operations. THE END