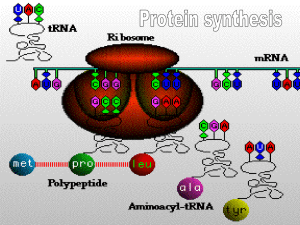
Outlines_Ch25
advertisement

Activating Transcription Chapter 25 25.1 Introduction 2 Figure 25.1 25.2 There Are Several Types of Transcription Factors • The basal apparatus determines the startpoint for transcription. • Activators determine the frequency of transcription. 3 Figure 25.2 • Activators work by making protein–protein contacts with the basal factors. • Activators may work via coactivators. • Some components of the transcriptional apparatus work by changing chromatin structure. 4 25.3 Independent Domains Bind DNA and Activate Transcription • DNA-binding activity and transcription-activation are carried by independent domains of an activator. • The role of the DNA-binding domain is to bring the transcription-activation domain into the vicinity of the promoter. Figure 25.3 5 25.4 The Two Hybrid Assay Detects Protein– Protein Interactions • The two hybrid assay works by requiring an interaction between two proteins: – one has a DNA-binding domain – the other has a transcriptionactivation domain 6 Figure 25.6 25.5 Activators Interact with the Basal Apparatus • The principle that governs the function of all activators is: – A DNA-binding domain determines specificity for the target promoter or enhancer. • The DNA-binding domain is responsible for localizing a transcription-activating domain in the proximity of the basal apparatus. • An activator that works directly has: – a DNA-binding domain – an activating domain 7 • An activator that does not have an activating domain may work by binding a coactivator that has an activating domain. Figure 25.7 8 • Several factors in the basal apparatus are targets with which activators or coactivators interact. Figure 25.8 9 • RNA polymerase may be associated with various alternative sets of transcription factors in the form of a holoenzyme complex. Figure 25.9 10 25.6 Some Promoter-Binding Proteins Are Repressors • Repression is usually achieved by affecting chromatin structure. – There are repressors that act by binding to specific promoters. 11 Figure 25.10 25.7 Response Elements Are Recognized by Activators • Response elements may be located in promoters or enhancers. Figure 25.11 12 • Each response element is recognized by a specific activator. • A promoter may have many response elements. – Elements may in turn activate transcription independently or in certain combinations. 13 25.8 There Are Many Types of DNA-Binding Domains • Activators are classified according to the type of DNA-binding domain. • Members of the same group have sequence variations of a specific motif that confer specificity for individual target sites. 14 25.9 A Zinc Finger Motif Is a DNA-Binding Domain • A zinc finger is a loop of ∼23 amino acids. – It protrudes from a zinc-binding site formed by His and Cys amino acids. • A zinc finger protein usually has multiple zinc fingers. 15 Figure 25.13 • The C-terminal part of each finger forms an αhelix. – The helix binds one turn of the major groove of DNA. • Some zinc finger proteins bind RNA instead of, or as well as, DNA. Figure 25.14 16 25.10 Steroid Receptors Are Activators • Steroid receptors are examples of ligandresponsive activators. – They are activated by binding a steroid (or other related molecules). • There are separate DNAbinding and ligandbinding domains. Figure 25.16 17 25.11 Steroid Receptors Have Zinc Fingers • The DNA binding domain of a steroid receptor is a type of zinc finger that has Cys but not His residues. • Glucocorticoid and estrogen receptors each have two zinc fingers. – The first determines the DNA target sequence. • Steroid receptors bind to DNA as dimers. 18 Figure 25.17 25.12 Binding to the Response Element Is Activated by Ligand-Binding • Binding of ligand to the C-terminal domain increases the affinity of the DNA-binding domain for its specific target site in DNA. Figure 25.19 19 25.13 Steroid Receptors Recognize Response Elements by a Combinatorial Code • A steroid response element consists of two short half sites that may be palindromic or directly repeated. • There are only two types of half sites. • A receptor recognizes its response element by the orientation and spacing of the half sites. 20 • The sequence of the half site is recognized by the first zinc finger. • The second zinc finger is responsible for dimerization, which determines the distance between the subunits. • Subunit separation in the receptor determines the recognition of spacing in the response element. 21 • Some steroid receptors function as homodimers, whereas others form heterodimers. • Homodimers recognize palindromic response elements. • Heterodimers recognize response elements with directly repeated half sites. Figure 25.20 Figure 25.21 22 25.14 Homeodomains Bind Related Targets in DNA • The homeodomain is a DNA-binding domain of 60 amino acids that has three α-helices. 23 Figure 25.24 • The C-terminal α-helix-3 is 17 amino acids and binds in the major groove of DNA. • The N-terminal arm of the homeodomain projects into the minor groove of DNA. • Proteins containing homeodomains may be either activators or repressors of transcription. 24 Figure 25.25 25.15 Helix-Loop-Helix Proteins Interact by Combinatorial Association • Helix-loop-helix proteins have a motif of 40 to 50 amino acids. – The motif comprises two amphipathic α-helices of 15 to 16 residues separated by a loop. • The helices are responsible for dimer formation. Figure 25.26 25 • bHLH proteins have a basic sequence adjacent to the HLH motif that is responsible for binding to DNA. • Class A bHLH proteins are ubiquitously expressed. – Class B bHLH proteins are tissue-specific. • A class B protein usually forms a heterodimer with a class A protein. 26 • HLH proteins that lack the basic region prevent a bHLH partner in a heterodimer from binding to DNA. • HLH proteins form combinatorial associations. – They may be changed during development by the addition or removal of specific proteins. Figure 25.27 27 25.16 Leucine Zippers Are Involved in Dimer Formation • The leucine zipper is an amphipathic helix that dimerizes. • The zipper is adjacent to a basic region that binds DNA. 28 • Dimerization forms the bZIP motif: – The two basic regions symmetrically bind inverted repeats in DNA. 29 Figure 25.28