ADDITION BY ME
CHAPTER 20 (GENES X)
PROMOTERS AND ENHANCERS
1
Eukaryotic gene control: purposes
and general principles
• Unlike bacterial cells and most single cell eukaryotes,
cells in multicellular organisms have relatively few
genes that are reversibly regulated by environmental
conditions
• However, gene control in multicellular organisms is
important for development and differentiation, and is
generally not reversible
2
Regulatory elements in eukaryotic DNA often
are many kilobases from start sites
• The basic principles that control transcription in bacteria also
apply to eukaryotic organisms: transcription is initiated at a
specific base pair and is controlled by the binding of transacting proteins (transcription factors) to cis-acting regulatory
DNA sequences
• However, eukaryotic cis-acting elements are often much
further from the promoter they regulate, and transcription
from a single promoter may be regulated by binding of
multiple transcription factors to alternative control elements.
3
Promoter sequence components can be
identified by:
Transcription control sequences can be identified by
analysis of a 5’-deletion series.
Finding precise consensus sequence that is bound by a
particular factor in multiple promoters.
Introduction of point mutations at particular base pairs and
testing the promoter function.
Finding the proteins which bound to promoters by
footprinting and mutation.
4
Transcription control
sequences can be identified
by analysis of a 5’-deletion
series.
5
REPORTER GENES
The primary objective is to identify gene regulatory sequences by testing
a large number of deletion and point mutants in an in vivo cell
transfection assays.
Promoter mapping studies can be done by creating artificial “reporter
gene” by fusing regulatory region of the test gene to a heterologous gene
coding sequences that direct the synthesis of a readily detectable protein
product.
The steady state level of the reporter protein in the transfected cell is
directly correlated to the steady state level of reporter mRNA, then it is
possible to use protein based reporter assays for promoter mapping
experiments.
6
Repoter gene constructs
When trying to determine what are the cis-acting regulatory regions
that control the expression (transcription) levels of a gene, is usually
routine to clone a large piece of DNA containing these sequences and
hook it to a downstream sequence that encodes an easy-to-measure
protein called a reporter.
This is a lot more convenient and accurate than trying to estimate the
absolute levels of mRNA.
In vivo transgene reporter expression driven by the promoter of
interest will reveal the temporal/spatial pattern of expression of the
gene, measured by an easier method than the alternative in situ
hybridization.
7
Reporter genes:
1.
must encode a protein activity that is similar to
one already present in the cell.
2.
The protein assay should be sensitive enough,
reproducible and easy to perform.
3.
The reporter protein function should not interfere
with host cellular processes in a way that will
alter intracellular signaling pathways or
metabolic rates.
8
Repoter gene constructs:
The most used reporters are:
bacterial b-galactosidase,
chloramfenicol acetyl transferase (CAT),
fire-fly luciferase
and most recently, the green fluorescent protein (GFP) which
allows measurements in living cells or organisms.
Part of the whole of a protein rather than a promoter can also
be fused in frame to this reporters to find out where it localizes.
Reporter gene must not being expressed endogenously in the
cell types that you are working on, so their background should
be null.
9
10
11
12
How does one identify transciptional
control mechanisms?
reporter gene (e.g. luciferase)
promoter
transcriptional unit
• promoter & transcriptional unit are independent
13
Use reporter to identify promoter sequence
reporter gene (e.g. luciferase)
1
promoter?
2
promoter?
3
promoter?
4
promoter?
5
promoter?
transcriptional unit
luciferase
activity
1
2
3
4
5
14
Use reporter to identify promoter sequence
promoter
= AAAATCACCCCACTGCAA
luciferase
activity
1
2
3
4
5
15
Purify Transcription Factor
Affinity chromatography
AAAATCACCCCACTGCAA
affinity
column
1) pour nuclear extract
2) allow to bind
3) elute transcription
factor
16
Transcriptional Reporter Gene Assay
Start site of Transcription
+1
-2000
Gene Promoter
Reporter Gene
ATG
LDL Receptor
Promoter
-141-- +36
Translational Start Site
Chloramphenicol Acetyltransferase
Luciferase
17
Construction and analysis of a 5-deletion series
18
Identification of transcription-control
elements with linker mutants
19
as
Using reporter protein to
determine the pattern
of a gene’s expression.
A)
In this example the
coding sequence for
protein X is replaced by
the coding sequence for
Y protein.
B)
B) Various fragments of
DNA containing
candidate regulatory
sequences are added in
combinations. The
recombinant DNA
molecules are then tested
for expression after their
transfection into a
variety of different cell
types of mammalian
cells, and the result is
summarized in C.
For experiments in eukaryotic
cells, two commonly
used reporter proteins
are enzymes bgalactosidase (bgal) and green
fluorescent protein or
20
GFP.
METHODS FOR STUDYING DNA-PROTEIN
INTERACTIONS
A. DNA footprinting
B. Electrophoretic mobility shift assay
C. Chromatin immunoprecipitation assay
21
DNA FOOTPRINTING
identify protein-DNA interactions
22
DNase I footprinting assays identify proteinDNA interactions
23
24
25
Electrophoretic mobility shift
assay
identify protein-DNA interactions
26
27
Gel-shift assays identify protein-DNA interactions
Fragment bound to
proteins are retarded
The free DNA fragment
migrate rapidly to the
bottom of the gel
28
ChIP (Chromatin
ImmunoPrecipitation)
Chromatin immunoprecipitation, or ChIP, refers to
a procedure used to determine whether a given
protein binds to a specific DNA sequence in vivo
29
DNA-binding proteins are crosslinked
to DNA with formaldehyde in vivo
Isolate the chromatin .Shear DNA
along with bound proteins into small
fragments.
Bind antibodies specific to the DNAbinding protein to isolate the complex by
precipitation .Reverse the cross-linking
to release the DNA and digest the
proteins.
Use PCR( Polymerase Chain Reaction )
to amplify specific DNA sequences to
see if they were precipitated with the
antibody
30
Chromatin immunoprecipitation
This methodology allows the identification
of sites in a genome that are occupied in
vivo by a gene regulatory protein. The
identities of the precipitated, amplified
DNA fragments can be determined by
hybridizing the mixture of fragments to
DNA microarrays.
31
ChIP: Chromatin immunoprecipitation
(formaldehyde)
Shearing of
DNA
Antibody against acetyl’ed
H3 & H4
Gene promoter is amplified using primers specific for that region
32
33
34
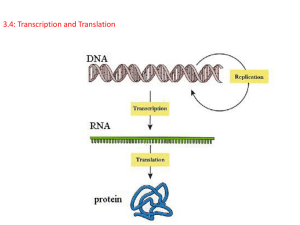
From DNA to RNA
Genes can be expressed with different efficiencies.
Gene A is transcribed and translated much more efficiently
than gene B. This allows the amount of protein A in the cell
to be much greater than that of protein B.
35
DNA is transcribed by the enzyme RNA polymerase. The RNA polymerase (pale blue) moves stepwise along the DNA,
unwinding the DNA helix at its active site. As it progresses, the polymerase adds nucleotides (here, small “T” shapes) one by
one to the RNA chain at the polymerization site using an exposed DNA strand as a template. The RNA transcript is thus a
single-stranded complementary copy of one of the two DNA strands. The polymerase has a rudder that displaces the newly
formed RNA, allowing the two strands of DNA behind the polymerase to rewind. A short region of DNA/RNA helix
(approximately nine nucleotides in length) is therefore formed only transiently, and a “window” of DNA/RNA helix
therefore moves along the DNA with the polymerase. The incoming nucleotides are in the form of ribonucleoside
triphosphates (ATP, UTP, CTP, and GTP), and the energy stored in their phosphate–phosphate bonds provides the 36
driving
force for the polymerization reaction (see Figure 5–4).
37
38
39
Cells produce several types of RNA
40
RNA polymerase I
rRNA transcription
RNA Polymerase II mRNA transcription
RNA Polymerase III tRNA and other small RNAs
Promoters
• RNA polymerase I and RNA Polymerase II are mostly
upstream of the start point.
• RNA Polymerase III lie downstream of the start point.
• Each promoter contains characteristic sets of short conserved
sequences that are recognized by the appropriate class of
factors.
RNA pol I and III recognize restricted set of promoters, rely
upon a small number of accessory factors.
RNA pol II show more variation in sequence, cis acting
elements are spread out over a region of >200bp.
41
Mammalian gene
Gene
Enhancer
Promoter Start Exon
Intron
Exon Termination
Promoter: DNA sequence located 5’ to a gene that is the sites
where transcription is initiated. Example: TATA box.
It is the binding sites for transcription factors.
Enhancer: Eukaryotic control element that can increase
expression of a gene. Located some distance from the gene
either up- or downstream.
Human gene number estimate: 28,000–34,000 genes.
42
Figure 24.01: Transcription is controlled by a promoter and enhancer.
43
Figure 24.02: RNA polymerase has >10 subunits.
44
RNA POLYMERASE I PROMOTER
• It transcribes genes for rRNA from a single type of promoter
• It consists of a bipartite sequence
• The core promoter and upstream control element (UCE)
The core promoter:
Surrounds the start point (-45 to +20)
It is sufficient to start transcription
UCE: -180 to -170
Both regions are rich in G.C bases
They are ~85% identical
45
RNA polymerase I requires two factors:
• The factor UBF1 wraps DNA around a protein structure to bring the core and UPE
into proximity.
• SL1 includes the factor TBP that is involved in initiation by all three RNA
polymerases.
• RNA polymerase binds to the UBF1-SL1 complex at the core promoter.
SL1 consists of 4 proteins:
3 TBP-associated factors (TAF1) + 1 TBP (TATA box binding protein)
TBP is necessary to start transcription for RNA polymerase II and III as well.
TBP is well conserved between species
TBP does not directly bind to G.C rich DNA. Other factors are also necessary.
46
Figure 24.05: Pol I promoters have two sequence components.
47
RNA Polymerase III promoter:
The promoters have two general classes
1) Promoters for 5S and tRNA genes are internal. They lie
downstream of the start point between positions +55
and +80 within the gene.
2) The promoters for snRNA genes lie upstream of the
start point.
3) There are two types of internal promoters: Type I and
Type II
Type I
Box A and Box C
Type II
Box A and Box B
48
There are three accessory factors:
TFIIIA Zinc finger protein
TFIIIB TBP and two proteins (B” and BRF proteins)
TFIIIC contains 5 subunit, >500 kD
TFIIIA and TFIIIC do not fully affect the initiation
reaction (assembly factors), their role is to bind TFIIIB
at the right location.
TFIIIB is crucial. It allows RNA pol III to bind at the
start site point (initiation factor or positioning factor).
49
• Internal promoters:
– have short consensus sequences
located within the transcription unit
– cause initiation to occur a fixed
distance upstream
• Upstream promoters contain
three short consensus sequences
upstream of the startpoint that
are bound by transcription
factors.
Figure 24.06: Pol III promoters may be downstream of startpoint.
50
5sRNA
tRNA
SnRNA
They increase the efficiency of transcription
Figure 24.07: There are three types of pol III promoters.
51
TFIIIB Is the Commitment Factor for Pol III Promoters
• TFIIIA and TFIIIC bind to
the consensus sequences
and enable TFIIIB to bind
at the startpoint.
• TFIIIB has TBP as one
subunit and enables RNA
polymerase to bind.
tRNA
Figure 24.08: Type 2 internal promoters use TFIIIC.
52
5sRN
A
Figure 24.09: Type 1 pol III promoters use TFIIIA/C.
Assembly factor (TFIIIA) ensure that TFIIIB (which includes TBP is bound just upstream of
the start point, to provide the positioning information.
53
The Startpoint for RNA Polymerase
II
• RNA polymerase II requires general
transcription factors (called TFIIX) to initiate
transcription.
• RNA polymerase II promoters have a short
conserved sequence Py2CAPy5 (the initiator
InR) at the startpoint.
54
• The TATA box is a common component of RNA polymerase II promoters
– It consists of an A-T-rich octamer located ~25 bp upstream of the startpoint.
• The DPE is a common component of RNA polymerase II promoters that do not
contain a TATA box.
• A core promoter for RNA polymerase II includes:
– the InR
– either a TATA box or a DPE
Figure 24.10
55
TBP Is a Universal Factor
• TBP is a component of the positioning
factor that is required for each type of
RNA polymerase to bind its promoter.
• The factor for RNA polymerase II is
TFIID, which consists of:
• TBP
• 11 TAFs
– The total mass is 800 kD.
Figure 24.11: Polymerases bind via commitment factors.
56
TBP Binds DNA in an Unusual Way
• TBP binds to the TATA box in the minor
groove of DNA.
• It forms a saddle around the DNA and bends it
by 80°.
• Some of the TAFs resemble histones and may
form a structure resembling a histone octamer.
57
TBP binds in the narrow groove and bends the DNA.
58
The conserved C-terminal domain of TBP binds to
TATA-box DNA
TBP
Start with binding of
TFIID which binds a
short DNA sequence
with many A-Ts TATA box
This binding causes a
dramatic distortion in
the DNA.
TBP is a subunit of
TFIID which binds the
TATA box.
Partially unwound
areas of TATA-Box
DNA
59
Most eukaryotic genes are regulated by
multiple transcription control
mechanisms
60
The basal apparatus assembles at the promoter
Binding of TFIID to the TATA box is the first step in
initiation.
Other transcription factors bind to the complex in a
defined order, extending the length of the protected
region on DNA.
When RNA polymerase II binds to the complex, it initiates
transcription.
61
RNA polymerase II requires general transcription factors
Initiation of transcription of a
eucaryotic gene by RNA
polymerase II.
To begin transcription, RNA
polymerase requires a number of
general transcription factors (called
TFIIA, TFIIB, and so on). (A) The
promoter contains a DNA sequence
called the TATA box, which is located
25 nucleotides away from the site at
which transcription is initiated. (B)
The TATA box is recognized and
bound by transcription factor TFIID,
which then enables the adjacent
binding of TFIIB (C).
62
. (D) The rest of the general transcription factors,
as well as the RNA polymerase itself, assemble at
the promoter. (E) TFIIH then uses ATP to pry
apart the DNA double helix at the transcription
start point, allowing transcription to begin.
TFIIH also phosphorylates RNA polymerase II,
changing its conformation so that the polymerase
is released from the general factors and can begin
the elongation phase of transcription. As shown,
the site of phosphorylation is a long C-terminal
polypeptide tail that extends from the polymerase
molecule. The assembly scheme shown in the
figure was deduced from experiments performed
in vitro, and the exact order in which the general
transcription factors assemble on promoters in
cells is not known with certainty. In some cases,
the general factors are thought to first assemble
with the polymerase, with the whole assembly
subsequently binding to the DNA in a single step.
The general transcription factors have been
highly conserved in evolution; some of those from
human cells can be replaced in biochemical
experiments by the corresponding factors from
simple yeasts.
63
General transcription factors assemble on
all promoters transcribed by RNA
polymerase II
Start with binding of TFIID which binds a
short DNA sequence with many A-Ts TATA box - typically 25 nucleotides
upstream from the transcription start site.
This binding causes a dramatic distortion in
the DNA.
Other factors assemble along with RNA
polymerase = transcription initiation
complex. TFIIH contains a protein kinase
subunit which adds phosphates to RNA
polymerase, releasing it from the complex
to start transcription
64
Figure 24.16: TFIIB helps position RNA polymerase II.
65
The basal / general transcription factors (core promoter factors):
TFIID: It has many subunits. TBP and TAFs
TBP is the only general transcription factor makes sequence-specific contacts with
DNA.
Within TFIID as a free protein complex, the factor TAFII230 binds to TBP and
controls the ability of TBP to bind to DNA.
The N-terminal domain of TAFII230 must be displaced from the DNA-binding
surface of TBP in order for TFIID to bind to DNA.
TFIIA: binds to TBP and stablilizes the TBP-DNA interaction. It also antogonizes
transcription repressors, it physically displaces or blocks several negative
transcriptional regulators from TFIID complex.
TFIIB: it is involved in the selection of transcription start sites, possibly setting
distances between promoters and transcription start sites. It interacts with diverse
activators that may serve to recruit TFIIB to the promoters.
66
TFIIF:
It binds tightly to RNA pol.II, suppresses nonspecific DNA
binding, and stabilizes the preinitiation complex.
It stimulates polymerase elongation rates by suppressing
transient pause during transcription.
Its subunit RAP74 makes contacts with DNA to position the
template during initiation. RAP74 has an ATP-dependent DNA
helicase activity. It affects the DNA topology, facilitates promoter
melting.
67
TFIIE: Interacts with TFIIH; involved in recruitment, stimulation of TFIIH and
promoter opening.
TFIIH: Its subunits have at least three enzymatic activities: DNA-dependent
ATPase, ATP-dependent helicase, and CTD kinase.
Two of its subunits have helicase activity (5’ to 3’ DNA helicase activity),
essential promoter opening and promoter escape.
TFIIH kinase/cyclin subcomplex: Cdks phosphorylate serines in the RNA
polymerase II CTD.
TFIIH kinase also functions as a Cdk activating kinase (CAK) and regulates
cell cycle transitions.
Some of its subunits required for nucleotide excision repair (transcriptional
coupled repair function).
68
The function of the CTD tail of RNA pol II
Most of the TFs are released before RNA
polymerase II leaves the promoter.
Phosphorylation of RNA pol II tail is needed
to release RNA pol II from the TFs so it can
make the transition to the elongation form.
CTD may coordinate processing of RNA
with transcription.
In mammals long polypeptide
tail of RNA pol II contains 52
repeats of the amino acid
sequence. The serine and
threonin side chains are
phosphorylated.
69
Figure 24.17: The CTD is phosphorylated at initiation.
The RNA refractory concept for
eucaryotic RNA polymerase II:
Not only does the polymerase
transcribe DNA into RNA, but it also
carries pre-mRNA-processing
proteins on its tails, which are then
transferred to the nascent RNA at the
appropriate time.
The capping enzyme
(Guanlylyl transferase), which
adds the G residues to the 5’ end
of the newly synthesized mRNA,
binds to phosphorylated CTD.
Some splicing factors bind to
CTD and also some components
of the cleavage/polyadenylation
70
apparatus.
Transcriptional coupled repair
The template strand of DNA in a transcribed
gene is preferentially repaired when DNA is
damaged.
What identifies the template strand of the DNA to
repair apparatus?
RNA polymerase stalls when it encounters the
damage in the template strand since it can not use
the damaged strand to direct complementary
base pairing.
Mfd gene has two roles:
• Displacing RNA pol. from DNA
• Causing the uvr ABC enzyme to bind to the
damaged DNA.
DNA is repaired by excision repair system.
RNA polymerase binds and produces a normal
transcript.
71
Chemically modified bases, such as thymine-thymine
dimers, are corrected by excision repair
72
Dimeric bases and
bulky lesions,
eg. large chemical
adducts,
are repaired by
Nucleotide Excision
Repair (NER)
73
Nucleotide Excision
Repair in Eucaryotes
Damage removed in the
context of a 24 - 28nt
(CSA and CSB)
fragment
Two pathways:
Global Genome repair
(Helicases)
(GG-NER)
Transcription coupled
repair (TC-NER)
Rereference:
de Laat W.L., Jaspers N.J.,Hoeijmakers J.H.
Genes & Development 13 2000
(SS-DNA binding protein)
74
In eucaryotes
TFIIH plays multiple roles.
The template strand of the transcribed
gene is preferentially repaired after UV
damage.
TFIIH.
TFIIH is found in alternative forms which
consists of a core associated with other
subunits.
TFIIH core protein and subunits
associated with other subunits that have
kinase activity and also a repair subunit.
TFIIH is required for initiation, but may
be replaced by other forms in response to
DNA damage.
TFIIH dissociates from RNA polymerase,
then reassociates at the site of DNA damage
with additional coupling components (i.e.
Rad26).
The repair function may require
75
modification or degradation of RNA pol.
Short sequence elements bind activators
Short conserved sequence elements are
dispersed in the region preceding the startpoint.
The upstream elements increase the
frequency of initiation.
The factors that bind to them to stimulate
transcription are called activators.
76
Promoters of RNA pol. II have short sequence elements.
TATA box
-30 bp
CAAT box -75 bp
GC box
-90 bp
Figure 24.21: The ß-globin promoter has three short sequence elements.
77
Figure 24.22: Promoters have mix and match modules.
78
Enhancers contain bidirectional elements that assist initiation
An enhancer activates the nearest promoter to it, and can be any distance either
upstream or downstream of the promoter.
Similar sequence elements are found in enhancers and promoters.
Enhancers form complexes of activators that interact directly or indirectly with
the promoter.
79
Enhancers Contain the Same Elements That Are
Found at Promoters
• Enhancers are made of the same short sequence elements that are found
in promoters.
• The density of sequence components is greater in the enhancer than in
the promoter.
80
Figure 24.24: Essential elements are more concentrated in enhancers.
The essential role of an enhancer is to increase the
concentration of transcription factors in the vicinity of the
promoter.
• An enhancer could change the overall
structure of the template by influencing the
density of the supercoil.
• It could be responsible for locating the
template at a particular place within the cell. i.e.
attaching it to the nuclear matrix.
• It provides an “entry site”, a point which RNA
pol. (or some other essential proteins) associate
with chromatin.
81
A model for gene activation
from an enhancer
General
transcription
factors usually can
not efficiently
initiate
transcription
alone.
Enhancers can be thousands of base pairs away, either upstream or
downstream and can either increase or decrease transcription.
“Action at a distance”
82
Enhancers work by increasing the concentration of
activators near the promoter
Enhancers usually work only in cis configuration
with a target promoter.
Enhancers can be made to work in trans
configuration by linking the DNA that contains the
target promoter to the DNA that contains the
enhancer via a protein bridge or by catenating the two
molecules.
The principle is that an enhancer works in any situation in
which it is constrained to be in the same proximity as the
promoter.
83
• The principle is that an enhancer works in any situation in
which it is constrained to be in the same proximity as the
promoter.
84
Figure 24.25: Enhancer activity requires proximity to the promoter.
Gene Expression Is Associated with
Demethylation
• Demethylation at the 5 end of the gene is
necessary for transcription.
85
CpG Islands Are Regulatory
Targets
• CpG islands surround the promoters of
constitutively expressed genes where they
are unmethylated.
• CpG islands also are found at the promoters
of some tissue-regulated genes.
86
Gene expression is associated with demethylation
• The state of methylation of DNA is controlled by
• methylases, which add methyl groups to the 5 position of
cytosine
• demethylases which remove the methyl group.
• There are two types of DNA methylase:
•
•
De novo methylase
modifies the DNA at a new position
recognizes DNA by the presence of a specific sequence
Maintenance methylase
acts on hemimethylated sites to convert to fully methylated
sites. It is a ubiquitious enzyme.
87
Formation of 5-methylcytosine occurs by
methylation of a cytosine base in the DNA
double helix. In vertebrates this event is
confined to selected cytosine nucleotides
located in the sequence CG.
88
Methylation pattern during embryogenesis:
In males:
• spermatocytes display methylation pattern characteristic
of mature sperm
• no further changes occur during spermatogenesis
• after fertilisation further changes occur
In females:
* oocytes mature through meiosis after birth and
methylation pattern is established.
89
Genes are inactive in gametes
Primordial germ cells develop in the embryo and lose all
their allelic differences irrespective of sex
(genome-wide demethylation)
Sex-specific pattern is imposed
During somatic development individual sequence-specific
methylation events occur in particular for genes to become
inactive.
90
How DNA methylation patterns are faithfully inherited:
In vertebrate DNAs a large fraction of the cytosine nucleotides in the sequence CG are
methylated. Because of the existence of a methly-directed methylating enzyme (the
maintenance methlytransferase), once a pattern of DNA methylation is established,
each site of methylation is inherited in the progeny DNA, as shown.
91
Gene expression is associated with
demethylation
92
CpG islands are regulatory targets
CpG islands surround the promoters of
constitutively expressed genes where they are
unmethylated.
They are also found at the promoters of some
tissue-regulated genes.
There are ~29,000 CpG islands in the human
genome.
Methylation of a CpG island prevents activation
of a promoter within it.
Repression is caused by proteins that bind to
methylated CpG doublets.
93
How DNA methylation may help to turn off
genes:
The binding of gene regulatory
machinery near an active promoter may
prevent DNA methylation by excluding
de novo methyltransferases. If most of
these proteins dissociated from the
DNA, however, as generally occurs
when a cell no longer produces the
required activator proteins, the DNA
becomes methylated, which enables
other proteins to bind, and these shut
down the gene completely by further
altering chromatin structure.
94
95
MspI cleaves CCGG
sequences both
methylated and
unmethylated.
HpaII cleaves
CCGG sequences
only nonmethylated
state.
Figure 24.26: Restriction enzymes differ
at methylated sites.
96
Restriction digests can analyze methylation
MspI cleaves CCGG sequences both methylated and unmethylated.
HpaII cleaves CCGG sequences only unmethylated state.
97
The DNA sequence given below is methylated in one type of tissue
but unmethylated in another type of tissue.
What sort of gel electrophoresis pattern would you obtain if you
cut the below DNA sequence isolated from different tissues with
these two enzymes?
98
Methylation status was checked by “methylation specific PCR (MSP)”
•
All unmethylated cytosines are deaminated and sulfonated, converting them to
uracils, while 5-methylcytosines remain unaltered.
(Sodium bisulfite converts unmethylated cytosine to uracil).
•
Thus, the sequence of the treated DNA will differ depending on whether the DNA
is originally methylated or unmethylated.
•
Also, the initially complementary DNA strands will no longer be complementary
after cytosine conversion.
•
Primers for use in MSP can be designed to specifically amplify either bisulfitesensitive, unmethylated strand or a bisulfite-resisant, methylated strand, based
upon these chemically-induced differences.
99
DNA treatment with Sodium Bisulfite
Unmethylated DNA
ggg
gcg
gac
cgc
g
Sodium bisulfite
modification
ggg
gug
gau
ugu
g
cmgcm
g
Methylated DNA
ggg
gcmg
gac
Sodium bisulfite
modification
ggg
gcmg
gau
cmgcm
g
100
Methylation specific PCR
The PCR primers are designed to specifically amplify the promoter
regions of the gene of interest.
If the DNA samples was originally unmethylated, an MSP reaction
product will be detectable when using primer set (labeled as “U”)
designed to be complementary to the unmethylated DNA sequence.
No product will be generated using primer set (labeled as
“M”) designed to be complementary to the derivative methylated
DNA sequence.
Conversely, an MSP product will be generated only using the M
primer set if the sample was originally methylated, and the U primers
will not amplify such a template.
101
• Methylation status was checked by “methylation specific PCR (MSP)”.
• Sodium bisulfite converts unmethylated cytosine to uracil
• 2 SETS OF PRIMERS are used in 2 SEPERATE PCR REACTIONS
• Cell lines expressing DLC-1 were found to be UNMETHYLATED.
102