Figure 2 - Algorithme Pharma
advertisement
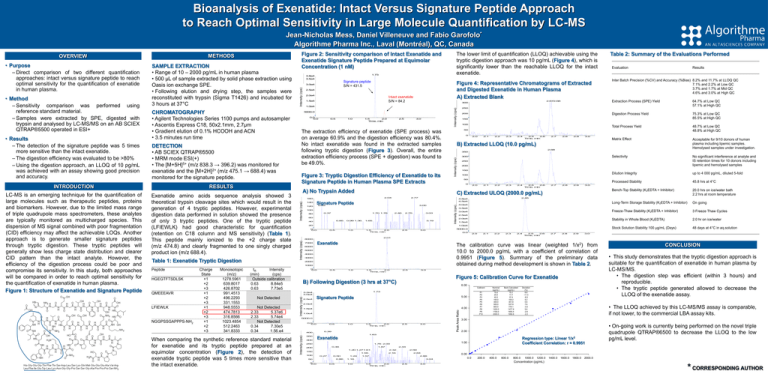
Bioanalysis of Exenatide: Intact Versus Signature Peptide Approach to Reach Optimal Sensitivity in Large Molecule Quantification by LC-MS Jean-Nicholas Mess, Daniel Villeneuve and Fabio Garofolo* Algorithme Pharma Inc., Laval (Montréal), QC, Canada 3.5e4 1.5e4 XIC of +MRM (2 pairs): 474.800/688.400 Da from Sample 10 (CR04-ZRA) of MSMS27.wiff (Turbo Spray), Smoothed 2.672.68 1.0e4 1.0 1.5 2.0 Time, min 2.5 3.0 Figure 3: Tryptic Digestion Efficiency of Exenatide to its Signature Peptide in Human Plasma SPE Extracts from Sam ple 40 (REF 25ug ACN) of Digestion.wiff (Turbo Spray) 2.04 Intensity, cps Intensity (cps) 160 140 M ax. 160.0 cps. 0.22 120 3.00 0.37 1.70 1.78 2.46 2.75 3.03 60 0.85 40 0.99 1.36 1.65 3.19 20 0.5 XIC of +M RM (10 pairs): 838.300/396.200 Da 1.0 1.5 2.0 Time, min 2.5 QMEEEAVR LFIEWLK NGGPSSGAPPPS-NH2 Monoisotopic (m/z) 1278.5961 639.8017 426.8702 991.4513 496.2293 331.1553 948.5553 474.7813 316.8566 1023.4854 512.2463 341.8333 Not Detected 2.33 5.37e6 2.33 5.74e4 Not Detected 0.34 7.30e5 0.34 1.56.e4 2.3 2.4 2.5 2.6 Time, min 2.7 2.8 2.9 Digestion Process Yield 75.3% at Low QC 85.5% at High QC Total Process Yield 48.7% at Low QC 48.8% at High QC Matrix Effect Acceptable for 9/10 donors of human plasma including lipemic samples. Hemolysed samples under investigation. Selectivity No significant interference at analyte and IS retention times for 10 donors including lipemic and hemolysed samples Dilution Integrity up to 4 000 pg/mL, diluted 5-fold Processed Stability 45.8 hrs at 4°C Bench-Top Stability (K2EDTA + Inhibitor) 20.0 hrs on ice/water bath 2.2 hrs at room temperature Long-Term Storage Stability (K2EDTA + Inhibitor) On going Freeze-Thaw Stability (K2EDTA + Inhibitor) 3 Freeze Thaw Cycles Stability in Whole Blood (K2EDTA) 2.0 hr on ice/water Stock Solution Stability 100 µg/mL (Days) 48 days at 4°C in aq.solution 3.0 B) Extracted LLOQ (10.0 pg/mL) Max. 6100.0 cps. 2.68 350 300 250 200 150 100 50 0 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Time, min 2.7 2.8 2.9 3.0 C) Extracted ULOQ (2000.0 pg/mL) XIC of +MRM (2 pairs): 474.800/688.400 Da from Sample 6 (CR04-P10A) of MSMS27.wiff (Turbo Spray) Max. 4.8e4 cps. 2.45 4.0e4 3.5e4 3.0e4 2.5e4 2.0e4 1.5e4 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Time, min 2.7 2.8 2.9 3.0 Exenatide 7000 The calibration curve was linear (weighted 1/x2) from 10.0 to 2000.0 pg/mL with a coefficient of correlation of 0.9951 (Figure 5). Summary of the preliminary data obtained during method development is shown in Table 2. 6000 5000 4000 3000 0 0.0 0.5 1.0 1.5 2.0 Time, min 2.5 3.0 Figure 5: Calibration Curve for Exenatide B) Following Digestion (3 hrs at 37°C) 6.00 Calibrant XIC of +M RM (10 pairs): 475.100/688.400 Da from Sam ple 63 (REF 25ug M eOH) of Digestion.wiff (Turbo Spray) 2.5e4 M ax. 3.2e4 cps. 1.73 P1 P2 P3 P4 P5 P6 P7 P8 P9 P10 5.00 Signature Peptide 2.0e4 4.00 1.5e4 1.0e4 5000.0 0.0 0.0 0.5 XIC of +M RM (10 pairs): 838.300/396.200 Da 400 1.0 1.5 2.0 Time, min 2.5 3.0 from Sam ple 63 (REF 25ug M eOH) of Digestion.wiff (Turbo Spray) M ax. 400.0 cps. 0.39 Nominal (pg/mL) 10.0 20.0 40.0 100.0 300.0 500.0 800.0 1400.0 1700.0 2000.0 Back-Calculated (pg/mL) 10.7 17.7 37.4 105.2 301.8 537.9 776.1 1477.9 1655.4 1956.8 Deviation (%) 6.6 -11.3 -6.5 5.2 0.6 7.6 -3.0 5.6 -2.6 -2.2 Intensity (cps) 350 300 2.00 0.46 200 1.87 1.20 1.271.61 Regression type: Linear 1/x2 Coefficient Correlation: r = 0.9951 1.00 1.76 2.00 • This study demonstrates that the tryptic digestion approach is suitable for the quantification of exenatide in human plasma by LC-MS/MS. • The digestion step was efficient (within 3 hours) and reproducible. • The tryptic peptide generated allowed to decrease the LLOQ of the exenatide assay. 3.00 Exenatide 250 CONCLUSION • The LLOQ achieved by this LC-MS/MS assay is comparable, if not lower, to the commercial LBA assay kits. 1.63 When comparing the synthetic reference standard material for exenatide and its tryptic peptide prepared at an equimolar concentration (Figure 2), the detection of exenatide tryptic peptide was 5 times more sensitive than the intact exenatide. 64.7% at Low QC 57.1% at High QC 2.01 3.2e4 3.0e4 Not Detected 2.2 M ax. 8160.0 cps. 2000 tR Intensity (min) (cps) Outside calibration 0.63 8.84e5 7.73e5 0.63 2.1 XIC of +MRM (2 pairs): 474.800/688.400 Da from Sample 11 (CR04-P1A) of MSMS27.wiff (Turbo Spray), Smoothed 0.0 from Sam ple 40 (REF 25ug ACN) of Digestion.wiff (Turbo Spray) Extraction Process (SPE) Yield 8000 Intensity (cps) HGEGTFTSDLSK Charge State +1 +2 +3 +1 +2 +3 +1 +2 +3 +1 +2 +3 2.0 5000.0 3.0 1000 Peptide 100 1.0e4 0 0.0 Inter Batch Precision (%CV) and Accuracy (%Bias) 8.2% and 11.7% at LLOQ QC 7.1% and 2.2% at Low QC 3.7% and 1.7% at Mid QC 4.6% and 3.0% at High QC 150 4.8e4 4.5e4 100 80 200 0 2.77 Signature Peptide Results 50 A) No Trypsin Added XIC of +M RM (10 pairs): 475.100/688.400 Da Evaluation 250 Intensity, cps (cps) Intensity 0.5 Table 2: Summary of the Evaluations Performed Max. 3296.5 cps. 300 Peak Area Ratio Table 1: Exenatide Tryptic Digestion Intact exenatide S/N = 84.2 The extraction efficiency of exenatide (SPE process) was on average 60.9% and the digestion efficiency was 80.4%. No intact exenatide was found in the extracted samples following tryptic digestion (Figure 3). Overall, the entire extraction efficiency process (SPE + digestion) was found to be 49.0%. Intensity, cps Exenatide amino acids sequence analysis showed 3 theoretical trypsin cleavage sites which would result in the generation of 4 tryptic peptides. However, experimental digestion data performed in solution showed the presence of only 3 tryptic peptides. One of the tryptic peptide (LFIEWLK) had good characteristic for quantification (retention on C18 column and MS sensitivity) (Table 1). This peptide mainly ionized to the +2 charge state (m/z 474.8) and clearly fragmented to one singly charged product ion (m/z 688.4). 2.0e4 0.0 0.0 Intensity (cps) LC-MS is an emerging technique for the quantification of large molecules such as therapeutic peptides, proteins and biomarkers. However, due to the limited mass range of triple quadrupole mass spectrometers, these analytes are typically monitored as multicharged species. This dispersion of MS signal combined with poor fragmentation (CID) efficiency may affect the achievable LOQs. Another approach is to generate smaller signature peptides through tryptic digestion. These tryptic peptides will generally show less charge state distribution and clearer CID pattern than the intact analyte. However, the efficiency of the digestion process could be poor and compromise its sensitivity. In this study, both approaches will be compared in order to reach optimal sensitivity for the quantification of exenatide in human plasma. Figure 1: Structure of Exenatide and Signature Peptide 2.5e4 5000.0 DETECTION • AB SCIEX QTRAP®5500 • MRM mode ESI(+) • The [M+5H]5+ (m/z 838.3 → 396.2) was monitored for exenatide and the [M+2H]2+ (m/z 475.1 → 688.4) was monitored for the signature peptide. RESULTS Figure 4: Representative Chromatograms of Extracted and Digested Exenatide in Human Plasma A) Extracted Blank Intensity, cps CHROMATOGRAPHY • Agilent Technologies Series 1100 pumps and autosampler • Ascentis Express C18, 50x2.1mm, 2.7µm • Gradient elution of 0.1% HCOOH and ACN • 3.5 minutes run time INTRODUCTION Signature peptide S/N = 431.5 3.0e4 Intensity, cps • Results – The detection of the signature peptide was 5 times more sensitive than the intact exenatide. – The digestion efficiency was evaluated to be >80% – Using the digestion approach, an LLOQ of 10 pg/mL was achieved with an assay showing good precision and accuracy. Max. 3.8e4 cps. 1.73 3.8e4 Intensity, cps • Method – Sensitivity comparison was performed using reference standard material. – Samples were extracted by SPE, digested with trypsin and analysed by LC-MS/MS on an AB SCIEX QTRAP®5500 operated in ESI+ XIC of +MRM (10 pairs): 475.100/688.400 Da from Sample 11 (NE) of Digestion.wiff (Turbo Spray) Intensity (cps) SAMPLE EXTRACTION • Range of 10 – 2000 pg/mL in human plasma • 500 µL of sample extracted by solid phase extraction using Oasis ion exchange SPE. • Following elution and drying step, the samples were reconstituted with trypsin (Sigma T1426) and incubated for 3 hours at 37°C The lower limit of quantification (LLOQ) achievable using the tryptic digestion approach was 10 pg/mL (Figure 4), which is significantly lower than the reachable LLOQ for the intact exenatide. Intensity, cps • Purpose – Direct comparison of two different quantification approaches: intact versus signature peptide to reach optimal sensitivity for the quantification of exenatide in human plasma. Figure 2: Sensitivity comparison of Intact Exenatide and Exenatide Signature Peptide Prepared at Equimolar Concentration (1 nM) Intensity (cps) METHODS Intensity Intensity,(cps) cps OVERVIEW • On-going work is currently being performed on the novel triple quadrupole QTRAP®6500 to decrease the LLOQ to the low pg/mL level. 2.44 2.32 2.59 150 1.55 100 0.27 0.50 0.86 50 0 0.0 1.24 2.16 2.84 1.57 0.00 2.88 1.16 3.04 0.0 200.0 400.0 600.0 800.0 1000.0 1200.0 1400.0 1600.0 1800.0 2000.0 Concentration (pg/mL) 0.5 1.0 1.5 2.0 Time, min 2.5 3.0 * CORRESPONDING AUTHOR