Overheads for Pat`s lecture

SIO 224
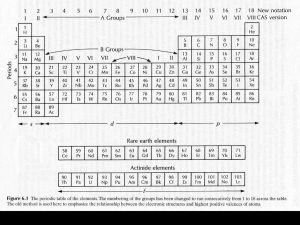
Models for bulk Earth, crust, mantle, and core composition
Composition of Earth cannot be understood in isolation
• Earth formation closely linked to sun and meteorite formation
• Nucleosynthesis in stars, from mainly H + He to other elements
Earth composition continued…..
Post-accretional chemical planetary processes
• shift from low-P to high-P processes on planets
• element segregation - grouping of elements, from cosmochemical to geochemical
Earth composition continued…..
lithophile elements (oxygen, oxides, silicate minerals,
Greek lithos - stone)
chacophile (sulphides, Greek khalkos=copper)
siderophile (metallic, Greek sideros=iron)
Earth composition composition…..
Goldschmidt’s classification is based on distribution in meteorites and Earth’s major geochemical reservoirs, but elements can still be further grouped based on their relative behavior in the Earth’s silicate portion, mantle and crust
Relative abundances:
• of the >100 known elements, only 90 occur naturally on Earth
• only 14 elements make up > 99% of the naturally occurring inorganic chemical compounds (minerals)
H, C, O, Na, Mg, Al, Si, P, S, K, Ca, Ti, Mn, and Fe
O, Mg, Si, Fe, Al, and Ca make up > 99% of the BSE
Normal igneous rock composition:
Major element > 1.0 wt. % of the rock or mineral
Minor element 0.1 - 1.0 wt. %
Trace element <0.1 wt. % (<1,000 ppm)
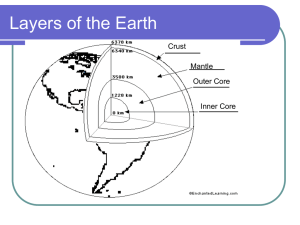
Core composition:
The Earth’s mantle:
How do we know the composition & mineralogy of the mantle?
• Cosmochemical constraints
• Geophysical constraints
• Experimental & theoretical constraints
• Direct samples of the mantle
- basalts
- crystalline samples
* alpine/orogenic peridotite
* abyssal peridotite
* ophiolite
* nodules/xenoliths
* xenoliths in/& kimberlite/lamproite
COSMOCHEMICAL
PREVIOUSLY…..
The six most abundant, nonvolatile rock-forming elements in the Sun are
Si (100), Mg (104), Fe (86), S (43), Al (8.4), and Ca (6.2). As the mantle of the Earth contains neither metal nor significant amounts of Fe 3+ , the sum of all oxides (by weight) must add up to 100%:
MgO + SiO
2
+ Al
2
O
3
+ CaO + FeO = 100% (1)
By inserting into Equation (1) average solar system abundance ratios, e.g., Si/Mg, Ca/Mg, and Al/Mg, one obtains
2:62 x MgO + FeO = 100% (2)
Considering that iron is distributed between mantle and core, the mass balance for iron can be written as
Fe core x 0.325 + Fe mantle x 0.675 = Fe total
(3) and similarly for magnesium, assuming a magnesium-free core,
Mg mantle x 0.675 = Mg total
(4)
By assuming that sulfur is quantitatively contained in the core and accounting for nickel in the core (Fe the core, Fe core total
/Ni = 17), the amount of iron in
, is calculated to be 75%. From Equations (2) to (4) and by using the solar abundance ratio for Fe total
/Mg total
, the hypothetical composition of the Earth’s mantle is obtained as:
Earth’s mantle solar model
MgO 35.8
SiO
2
51.2
FeO 6.3
Al
2
O
3
3.7
CaO 3.0
DIRECT SAMPLES (Peridotite)
Common mantle minerals:
• Olivine
• Orthoppyroxene
• Clinopyroxene
• Spinel
• Garnet
Mineralogy of the source
Pyrolite: hypothetical mixture of of residual mantle material
(xenolith) + primitive basaltic magma
Note:
All peridotites are metamorphic rocks that have had complex subsolidus history after melt extraction ceased - strain, crystal segregation, deformation, metasomatism, etc. Thus peridotites show compositional variations, particularly in their trace element contents. Nevertheless, they show definite and coherent trends - the least-depleted peridotites (lowest MgO, but highest
CaO, Al
2
O
3 and other incompatible trace elements that partition into the liquid phase during partial melting (i.e., fertile) plot closest to the composition of the primitive mantle (PM).
Trace element content of the PM has also been estimated basically following similar assumptions and arguments used for the majors.
HSE (Os, Ir, Pt, Ru, Rh, Pd, Re, Au) are low in the Earth’s mantle, but not low enough as expected - hence the “late veneer” hypothesis..
Mantle samples
Composition of the mantle of the Earth assuming average solar system element ratios for the whole Earth versus PM mantle compositions
Ref. solar model (1) (2) (3) (4) (5) (6) (7) (8)
MgO 35.8
36.77
38.1 38.3 36.8 35.5 37.8 37.8 37.77
Al
2
O
3
3.7
4.49
3.3 4.0 4.1 4.8 4.06 4.4 4.09
SiO
2
51.2
CaO 3.0
45.40 45.1
3.65 3.1
45.1
3.5
45.6
3.5
46.2
4.4
46.0
3.27
45.0
3.5
46.12
3.23
FeO t 6.3
8.10 8.0 7.8 7.5 7.7 8.1 7.49
Total 100 98.41 97.6 98.7 97.5 98.6 98.8 98.7
Mg#, molar Mg/(Mg+Fe); FeOt, all Fe as FeO; (RLE/Mg)N, refractory lithophile elements normalized to Mg- and CI-chondrites. References:
(1) Palme & ONeil’04 (2) Ringwood’79 = “pyrolite” model (3) Jagoutz et al.’79 (4) Wa ¨ nke et al.’84 (5) Palme & Nickel’85 (6) Hart &
Zindler’86 (7) McDonough & Sun’95 (8) Alle`gre et al.’95
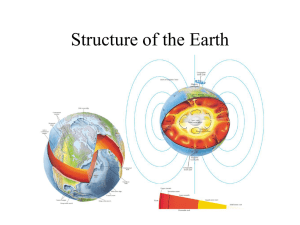
GEOPHYSICAL & EXPERIMENTAL CONSTRAINTS
Pressure increases with depth:
P =
gh; for the upper few hundred km,
= 3.3 g/cc
= 0.33 kbar h, where h is in km.
Is the mantle compositionally layered or not………
Oceanic basalts as probes of the upper mantle
Adiabatic decompression partial melting of the mantle:
Intraplate magmatism: linear island chains & LIPs
Partial melting of the mantle (intraplate setting):
Typical normalized element patterns of terrestrial igneous rocks
Convergent margin magmatism
Partial Melting of the mantle (subduction zone setting):
Major elements of island arc volcanic rocks & magma series
IAB : MORB:
MgO <
(Mg#)<
K
2
O >
Al
2
O
3
>
(although variable & some C-A basalts have 17-20% (high-Al basalts), some believe that these are parental to C-A series rocks)
Trace elements of island arc volcanic rocks & magma series
(continued)
Note enrichment in LIL and depletion in HFS
Several potential source components for island arc magmas
Continental crust
From Rudnick & Gao, 2005
Continents ( early studies):
•an average intermediate or andesitic composition
•only 0.6% by mass of BSE, but 20-70% of incomp. elements
•contains the oldest rocks (4.0 Ga Acasta gneiss) & minerals
(4.4 Ga detrital zircon) = rich geological history
•seismically divided into
•upper- [granodiorite]
•deep-
•middle-
•lower-crust [high-grade metamorphic rocks & granulite xenoliths; increasing metamorphic grade & mafic rx = more mafic?]
•vertically stratified and laterally heterogeneous
Upper crust composition:
- weighted averages of exposed rocks
- averages of finegrained sediments or glacial deposits
Deep crust composition:
(1) samples from deep crust
(2) seismic velocities
(3) heat flow measurements
Comparison of (a) REE &
(b) additional trace element compositions of the upper, middle and lower crust recommended by Rudnick & Gao ‘05.
Middle crust metasedimentary rocks, but dominated by DTTG
Deep crust mafic
Comparison of (a) REE &
(b) additional trace element compositions of the bulk crust - this study = Rudnick
& Gao, ‘05.
Bulk cc composition:
•intermediate
•high Mg#
•up to 50% of BSE’s inc. el.
•depleted in Nb relative to La
•enriched in Pb
•subchondritic Nb/Ta
If the crust grows ultimately by igneous processes, then the disparity between crust and primary mantle melt compositions requires additional process(es) such as:
1) Recycling of mafic/ultramafic lower crust and upper mantle (density foundering or delamination)
2) Mixing silicic melts from subducted slab and mafic melt from mantle peridotite (e.g., Archean DTTG)
3) Weathering of the crust, with preferential recycling of Mg
& Ca into the mantle via subduction (not supported by observation)
4) Ultramafic cumulates complementary to andesitic crust are present in the upper mantle
But Nb depletion suggests that ~80% of the crust was generated in a convergent margin
Major elements of island arc volcanic rocks & magma series
IAB : MORB:
MgO <
(Mg#)<
K
2
O >
Al
2
O
3
>
(although variable & some C-A basalts have 17-20% (high-Al basalts), some believe that these are parental to C-A series rocks)
Selected references:
Ringwood, A.E., 1979, Origin of the Earth and Moon,
Springer-Verlag, N.Y., 295 p.
Holland, H.D. and Turekian, K.K., 2003, Treatise on
Geochemistry, vol. 1, Meteorites, Comets and Planets.
Stevenson, D.J., 1981, Models of the Earth’s Core,
Science 214, #4521, 611-619.