Selenium and Tellurium Chemistry
advertisement

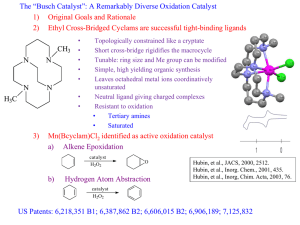
Carl Trudel, Literature Meeting Wednesday, April 11th 2012 About this presentation Singh, F. V.; Wirth, T. In Organoselenium Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA, 2012, p 321-360. Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 2 About Me 3 Presentation Schedule Some selenium facts Stoichiometric reactions Selenium as a catalyst Carbonylation Oxidation (B.-V., epoxidation, selenylation-deselenylation, alkyne, allylic, alcohol, imine, aniline...) Halobromation GPx like activity Alkylation Selenium as a ligand for Copper Palladium 4 Fun Facts Discovered by J. J. Berzelius in 1817. Selenium => Selene (moon) Chalcogen (O, S, Te) Among the 25 least common elements 0.05 – 0.09 ppm in the earth crust Recommended daily intake: 55µg (max 400µg/day) >1000µg/day => intoxications Brazil nuts, fishes and seafood (oyster and tuna)... North American cereals (Beer!) Berzelius, J. J. Afhandl. Fys. Kemi Mineralogi. 1818, 42. Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 285-302. 5 http://www.passeportsante.net/fr/Solutions/PlantesSupplements/Fiche.aspx?doc=selenium_ps [April 2012] Other Facts and Nomenclature Used in everyday applications Glass-making, electronics, printers, solar cells Glutathione peroxidase enzymes and selenoproteines Antioxidants, antitumor, antimicrobial, antiviral Se(s) 44.84 $/mol SeO2 54.59 $/mol Ph2Se 4 768.33 $/mol (PhSe)2 3 970,29 $/mol [mCPBA 120.11 $/mol] 6 www. sigmaaldrich.com [april 2012] Soichiometric Selenium Chemistry Reich, H. J.; Cohen, M. L.; Clark, P. S. Org. Synth. 1988, 50-9, 533-537. Thompson, D. P.; Boudjouk, P. J. Org. Chem. 1988, 53, 2109-2112. 7 Santi, C.; Wirth, T. Tetrahedron: Asymm. 1999, 10, 1019-1023. 1st Selenium Catalyzed Reaction Carbonylation of aminoalcohols 8 Sonoda, N.; Yamamoto, G.; Natsukawa, K.; Kondo, K.; Murai, S. Tetrahedron Lett. 1975, 16, 1969-1972. Selenium Based Oxygen Transfer Reagents Perseleninic acid Hydroxy Perhydroxy Selenane 9 Davis, F. A.; Reddy, R. T. J. Org. Chem. 1992, 57, 2599-2606. Baeyer-Villiger Reaction Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 10 Baeyer-Villiger Reaction, Perseleninic Acids Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 11 Catalytic Baeyer-Villiger Reaction Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 12 Catalytic Baeyer-Villiger Reaction 13 ten Brink, G.-J.; Vis, J.-M.; Arends, I. W. C. E.; Sheldon, R. A. J. Org. Chem. 2001, 66, 2429. Catalytic Baeyer-Villiger Reaction C3° > C2° > Bn > Ar/H* > C1° > Me CF3CH2OH, 20 °C Hydrolysis might be an issue Important substituent effect 14 ten Brink, G.-J.; Vis, J.-M.; Arends, I. W. C. E.; Sheldon, R. A. J. Org. Chem. 2001, 66, 2429. Seleninic Acid Epoxidation Pioneer work by Sharpless 15 Hori, T.; Sharpless, K. B. J. Org. Chem. 1978, 43, 1689-1697. Seleninic Acid Epoxidation Pioneer work by Sharpless 16 Hori, T.; Sharpless, K. B. J. Org. Chem. 1978, 43, 1689-1697. Seleninic Acid Epoxidation DCM or trifluoroethanol Recyclable perfluorinated solvent 30 % H2O2 causes emulsions Dihydroxylation NaOAc increase yields Betzemeier, B.; Lhermitte, F.; Knochel, P. Synlett 1999, 489. Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin 17 Heidelberg, 2011, p 251-283. Seleninic Acid Dihydroxylation Sheldon, R. A. et al. J. Chem. Soc., Perkin Trans. 1 2001, 224. Santoro, S.; Santi, C.; Sabatini, M.; Testaferri, L.; Tiecco, M. Adv. Synth. Catal. 2008, 350, 2881-2884. 18 Sequential Selenylation-Desenylation Santi, C. Et al. Chem. Eur. J. 2002, i, 1118. Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem. Int. Ed. 2009, 48, 8409. 19 Sequential Selenylation-Desenylation Santi, C. Et al. Chem. Eur. J. 2002, i, 1118. Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem. Int. Ed. 2009, 48, 8409. 20 Sequential Selenylation-Desenylation Santi, C. Et al. Chem. Eur. J. 2002, i, 1118. Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem. Int. Ed. 2009, 48, 8409. 21 Alkyne Oxidation 22 Santoro, S.; Battistelli, B.; Gjoka, B.; Si, C.-w. S.; Testaferri, L.; Tiecco, M.; Santi, C. Synlett, 2010, 1402. Alkyne Oxidation 23 Santoro, S.; Battistelli, B.; Gjoka, B.; Si, C.-w. S.; Testaferri, L.; Tiecco, M.; Santi, C. Synlett, 2010, 1402. Alkyne Oxidation 24 Santoro, S.; Battistelli, B.; Gjoka, B.; Si, C.-w. S.; Testaferri, L.; Tiecco, M.; Santi, C. Synlett, 2010, 1402. Alcohol Oxidation 25 van der Toorn, J. C.; Kemperman, G.; Sheldon, R. A.; Arends, I. W. C. E. J. Org.Chem. 2009, 74, 3085. Alcohol Oxidation Excess of TBHP is to be avoided Presence of water decrease the selectivity Preactivation of the catalyst shortens reaction time van der Toorn, J. C.; Kemperman, G.; Sheldon, R. A.; Arends, I. W. C. E. J. Org.Chem. 2009, 74, 3085. 26 Alcohol Oxidation 27 van der Toorn, J. C.; Kemperman, G.; Sheldon, R. A.; Arends, I. W. C. E. J. Org.Chem. 2009, 74, 3085. Alcohol Oxidation 28 Ehara, H.; Noguchi, M.; Sayama, S.; Onami, T. J. Chem. Soc., Perkin Trans. 1 2000, 1429. Allylic Oxidation of Alkene 29 Crich, D.; Zou, Y. Org. Lett. 2004, 6, 775-777. Allylic Oxidation of Alkene Iodoxybenzene (H2O2 less selective) Electron-rich alkenes preferentially Stable catalyst Diselenide is recovered after Na2S2O5 quench(86 - 92%) 30 Crich, D.; Zou, Y. Org. Lett. 2004, 6, 775-777. Allylic Oxidation of Alkene Oxidation on the more highly substituted side Endocyclic oxidation for 1-substituted cyclohexene krel: CH2 > CH3 > CH Follows Bredt’s rule Crich, D.; Zou, Y. Org. Lett. 2004, 6, 775-777. Smith, M. B. Organic Synthesis; McGraw-Hill: Boston, MA, 2002; pp. 273-275. 31 Imine Oxidation, Catalytic Hydroxylation 32 Brodsky, B. H.; Du Bois, J. J. Am. Chem. Soc. 2005, 127, 15391. Aniline Oxidation Priewisch, B.; Rück-Braun, K. J. Org. Chem. 2005, 70, 2350-2352. Zhao, D.; Johansson, M.; Bäckvall, J.-E. Eur. J. Org. Chem. 2007, 4431. 33 Oxidation of Bromide Salts Br2, Br3+, HOBr Seleninic acid electron rich reacts faster Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 34 Oxidation of Bromide Salts, Seleninic Acids Unknown brominating species Electron donating group acceleration Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 35 Oxidation of Bromide Salts, Selenoxide 36 Goodman, M. A.; Detty, M. R. Organomet. 2004, 23, 3016. Oxidation of Bromide Salts, Seleninic Acid 37 Drake, M. D.; Bateman, M. A.; Detty, M. R. Organomet. 2003, 22, 4158. Disulfide Formation Gluthathione peroxidase (GPx) Selenoenzyme (L-selenocysteine) Reactive oxygen species Neurodegenerative disease (Parkinson, Alzheimer), physiological and inflammatory processes. Chalcogen-based catalytic antioxidants Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 38 GPx Activity Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 39 Catlytic Reduction of Enones 40 Tian, F.; Lu, S. Synlett 2004, 1953. Catalytic Disulfide Formation Alberto, E. E.; Braga, A. L.; Woollins, J. D.; Laitinen, R. In Selenium and Tellurium Chemistry; Springer Berlin Heidelberg, 2011, p 251-283. 41 Diethyl Zinc Addition to Aldehydes 42 Santi, C.; Wirth, T. Tetrahedron: Asym. 1999, 10, 1019-1023. Wirth, T. Tetrahedron Lett. 1995, 36, 7849-7852. Diethyl Zinc Addition to Aldehydes 43 Santi, C.; Wirth, T. Tetrahedron: Asym. 1999, 10, 1019-1023. Wirth, T. Tetrahedron Lett. 1995, 36, 7849-7852. Diethyl Zinc Addition to Aldehydes 44 Braga, A. L.; Galetto, F. Z.; Rodrigues, O. E. D.; Silveira, C. C.; Paixão, M. W. Chirality 2008, 20, 839-845. Diethyl Zinc Addition to Enones 45 Shi, M.; Wang, C.-J.; Zhang, W. Chem. Eur. J. 2004, 10, 5507-5516. Diethyl Zinc Addition to Enones 46 Shi, M.; Wang, C.-J.; Zhang, W. Chem. Eur. J. 2004, 10, 5507-5516. Malonate Alkylation 47 Braga, A. L.; Galetto, F. Z.; Rodrigues, O. E. D.; Silveira, C. C.; Paixão, M. W. Chirality 2008, 20, 839. Malonate Alkylation 48 Braga, A. L.; Galetto, F. Z.; Rodrigues, O. E. D.; Silveira, C. C.; Paixão, M. W. Chirality 2008, 20, 839. Conclusion Selenium compounds are very versatile catalysts Different oxidation state allows completely different reaction pathways Little work as been focusing on their strong electron donating properties as a ligand Little success in achieving stereoselective reactions with catalytic amount of enantioenriched organoselenium Developpement towards its industrial use rather than fine chemistry 49