Instrument calibration methods
advertisement

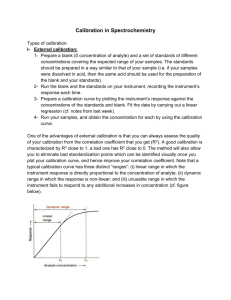
Calibration methods Chemistry 243 Figures of merit: Performance characteristics of instruments Precision Accuracy Selectivity Sensitivity Limit of Detection Limit of Quantitation Dynamic Range Precision vs. Accuracy in the common verbiage (Webster’s) Precision: Accuracy: The quality or state of being precise; exactness; accuracy; strict conformity to a rule or a standard; definiteness. The state of being accurate; exact conformity to truth, or to a rule or model; precision. These are not synonymous when describing instrumental measurements! Precision and accuracy in this course Precision: Degree of mutual agreement among data obtained in the same way. Accuracy: Measure of closeness to accepted value Absolute and relative standard deviation, standard error of the mean, coefficient of variation, variance. Extends in between various methods of measuring the same value Absolute or relative error Not known for unknown samples Can be precise without being accurate!!! Precisely wrong! Precision - Metrics Most important Often seen as % Handy, common Sensitivity vs. Limit of Detection NOT THE SAME THING!!!!! Sensitivity: Ability to discriminate between small differences in analyte concentration at a particular concentration. calibration sensitivity—the slope of the calibration curve at the concentration of interest Limit of detection: Minimum concentration that can be detected at a known confidence limit Typically three times the standard deviation of the noise from the blank measurement (3s or 3s is equivalent to 99.7% confidence limit) Such a signal is very probably not merely noise Calibration Curve, Limit of Detection, Sensitivity Signal Sensitivity* = Slope *Same as Working Curve **Not improved by amplification alone S/N = 3 0 0 LOD Analyte Mass or Concentration Selectivity Degree to which a method is free from interference from other contaminating signals in matrix S mAc A mB cB mC cC No measurement is completely free of interferences mB Selectivity coefficient: kB, A mA Calibration Curves: Sensitivity and LOD Signal For a given sample standard deviation, s, steeper calibration curve means better sensitivity Insensitive to amplification S/N = 3 0 0 LOD LOD Analyte Mass or Concentration Dynamic range The maximum range over which an accurate measurement can be made From limit of quantitation to limit of linearity LOQ: 10 s of blank LOL: 5% deviation from linear Ideally a few logs Absorbance: 1-2 MS, Fluorescence: 4-5 NMR: 6 Calibration Curves: Dynamic Range and Noise Regions Signal Calibration Curve becomes poor above this amount of analyte Poor Quant Noise Region Dynamic Range S/N = 3 0 0 LOD LOQ LOL Analyte Mass or Concentration Types of Errors Random or indeterminate errors Systematic errors Handled with statistical probability as already shown Instrumental errors Personal errors Method errors Gross errors Human error Careless mistake, or mistake in understanding Often seen as an outlier in the statistical distribution “Exactly backwards” error quite common Systematic errors Present in all measurements made in the same way and introduce bias. Instrumental errors Personal errors Wacky instrument behavior, bad calibrations, poor conditions for use Electronic drift, temperature effects, 60Hz line noise, batteries dying, problems with calibration equipment. Originate from judgment calls Reading a scale or graduated pipette, titration end points Method errors Non-ideal chemical or physical behavior Evaporation, adsorption to surfaces, reagent degradation, chemical interferences Instrument calibration Determine the relationship between response and concentration Calibration curve or working curve Calibration methods typically involve standards Comparison techniques External standard* Standard addition* Internal standard* * calibration curve is required External standard calibration (ideal) External Standard – standards are not in the sample and are run separately Generate calibration curve (like PS1, #1) Run known standards and measure signals Plot vs. known standard amount (conc., mass, or mol) Linear regression via least squares analysis Compare response of sample unknown and solve for unknown concentration All well and good if the standards are just like the sample unknown External standard calibration (ideal) Sample Unknown Signal External Calibration Standards including a blank Sample Unknown Amount S/N = 3 0 0 LOD Analyte Mass or Concentration In class example of external standard calibration Skoog, Fig. 13-13 b pathlength c concentration Sample Unknown Signal P0 A log bc P molar absorptivity External Calibration Standards including a blank Sample Unknown Amount S/N = 3 0 0 LOD Analyte Mass or Concentration Real-life calibration Subject to matrix interferences Matrix = what the real sample is in pH, salts, contaminants, particulates Glucose in blood, oil in shrimp Concomitant species in real sample lead to different detector or sensor responses for standards at same concentration or mass (or moles) Several clever schemes are typically employed to solve real-world calibration problems: Internal Standard Standard Additions Internal standard A substance different from the analyte added in a constant amount to all samples, blanks, and standards or a major component of a sample at sufficiently high concentration so that it can be assumed to be constant. Plotting the ratio of analyte to internal-standard as a function of analyte concentration gives the calibration curve. Accounts for random and systematic errors. Difficult to apply because of challenges associated with identifying and introducing an appropriate internal standard substance. Similar but not identical; can’t be present in sample Lithium good for sodium and potassium in blood; not in blood Standard additions Classic method for reducing (or simply accommodating) matrix effects Especially for complex samples; biosamples Often the only way to do it right You spike the sample by adding known amounts of standard solution to the sample Have to know your analyte in advance Assumes that matrix is nearly identical after standard addition (you add a small amount of standard to the actual sample) As with “Internal Standard” this approach accounts for random and systematic errors; more widely applicable Must have a linear calibration curve How to use standard additions To multiple sample volumes of an unknown, different volumes of a standard are added and diluted to the same volume. Fixed parameters: cs = Conc. of std. – fixed Vt = Total volume – fixed Vx = Volume of unk. – fixed cx = Conc. of unk. - seeking Calibration Standard (Fixed cs) Vx Vx Vx Vx Vs1 Vs2 Vs3 Vs4 Vt Vt Vt Vt Non-Fixed Parameter: Vs = Volume of std. – variable Volume top-off step: Vx diluted to Vt Vs diluted to Vt How to use standard additions To multiple sample volumes of an unknown, different volumes of a standard are added and diluted to the same volume. 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑆𝑠𝑡𝑑 + 𝑆𝑥 Combined Signal S1 S2 S3 S4 0 0 Concentration How to use standard additions 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑆𝑠𝑡𝑑 + 𝑆𝑥 𝑆𝑠𝑡𝑑 = 𝑘 ∙ 𝑓𝑑𝑖𝑙 ∙ 𝑐𝑠𝑡𝑑 𝑘𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 = 𝑉𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑥 𝑐𝑥 𝑆𝑥 = 𝑘 ∙ 𝑓𝑑𝑖𝑙′ ∙ 𝑐𝑥 = 𝑉𝑡𝑜𝑡𝑎𝑙 Combined Signal k = slope or sensitivity 0 0 Concentration How to use standard additions 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑆𝑠𝑡𝑑 + 𝑆𝑥 𝑆𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 𝑘𝑉𝑥 𝑐𝑥 = + 𝑉𝑡𝑜𝑡𝑎𝑙 𝑉𝑡𝑜𝑡𝑎𝑙 How to use standard additions 𝑆𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 𝑘𝑉𝑥 𝑐𝑥 = + 𝑉𝑡𝑜𝑡𝑎𝑙 𝑉𝑡𝑜𝑡𝑎𝑙 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑚𝑉𝑠𝑡𝑑 + 𝑏 𝑘𝑐𝑠𝑡𝑑 𝑚= 𝑉𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑥 𝑐𝑥 𝑏= 𝑉𝑡𝑜𝑡𝑎𝑙 Remember, Vstd is the variable. Knowns: cstd Vtotal Vx How to use standard additions 𝑘𝑐𝑠𝑡𝑑 𝑚= 𝑉𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑥 𝑐𝑥 𝑏= 𝑉𝑡𝑜𝑡𝑎𝑙 S, Combined Signal 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑚𝑉𝑠𝑡𝑑 + 𝑏 Get m (slope) and b (intercept) from linear least squares 0 0 How do I handle k ? Vs Determine cx via standard curve extrapolation … 𝑆𝑡𝑜𝑡𝑎𝑙 𝑘𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 𝑘𝑉𝑥 𝑐𝑥 = + 𝑉𝑡𝑜𝑡𝑎𝑙 𝑉𝑡𝑜𝑡𝑎𝑙 At the x-intercept, S = 0 𝑘𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 𝑘𝑉𝑥 𝑐𝑥 =− 𝑉𝑡𝑜𝑡𝑎𝑙 𝑉𝑡𝑜𝑡𝑎𝑙 𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 = −𝑉𝑥 𝑐𝑥 Skoog, Fig. 1-10 𝑉𝑠𝑡𝑑 0 𝑐𝑠𝑡𝑑 𝑐𝑥 = − 𝑉𝑥 Seeking [analyte] known known Vstd when S = 0 … or determine cx by directly using fit parameters 𝑉𝑠𝑡𝑑 𝑐𝑠𝑡𝑑 𝑐𝑥 = − 𝑉𝑥 𝑆𝑡𝑜𝑡𝑎𝑙 = 𝑚𝑉𝑠𝑡𝑑 + 𝑏 = 0 𝑚𝑉𝑠𝑡𝑑 = −𝑏 Final calculation: 𝑉𝑠𝑡𝑑 𝑏 =− 𝑚 All knowns 𝑏 − 𝑐 𝑚 𝑠𝑡𝑑 𝑏𝑐𝑠𝑡𝑑 𝑐𝑥 = − = 𝑉𝑥 𝑚𝑉𝑥 … in conclusion, an easy procedure to perform and interpret; you take values you know and do a linear least squares fit to get m and b