Caroline Fairhurst (MS PowerPoint , 1824kb)
advertisement

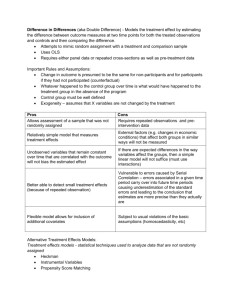
Treatment Switching in the VenUS IV trial Methods to manage treatment non-compliance in RCTs with time-to-event outcomes Caroline Fairhurst York Trials Unit Context • • • • Two arm RCT Clinical setting Continuous treatment Time-to-event outcome (e.g., death, healing) Dream or reality? Ideal Reality • All participants will remain in the trial throughout follow-up • Participants withdraw from the trial and are lost to follow-up • Will be concordant with their allocated treatment • Withdraw from treatment • Will provide outcome data • Deviate from their allocated trial treatment Treatment switching Problem? • Switching to the alternative trial treatment makes randomised groups more similar • Dilutes the treatment effect observed from a comparison of treatment groups as randomised ignoring deviations from allocated treatment (ITT) • If you want to estimate the effect had fewer switches occurred, ITT analysis biased towards the null of no difference VenUS IV trial Venous leg ulcers are wounds that form on gaiter region of the leg They are painful, malodorous and prone to infection Difficult to heal and 12 month recurrence rates are 18-28% VenUS I, II, III Four layer bandaging is current gold standard VenUS IV trial • Population: Patients aged over 18 with at least one venous leg ulcer and able to tolerate high compression to the leg • Intervention: Two layer high compression hosiery • Control: Four layer high compression bandaging • Outcome: Time to healing of the largest ulcer Treatment switching Randomised n=457 Hosiery n=230 Non-trial treatment n=42 n=16 Hosiery Bandage n=224 n=46 Bandage Non-trial treatment n=46 Treatment switching Simple methods - ITT Intention-to-treat • ITT recommended (ICH E9) • Compares individuals in the treatment groups to which they were randomised • Estimates the effect of offering the two treatment policies to patients with whatever subsequent changes may occur • “pragmatic effectiveness not biological efficacy” • But what about effect of receiving experimental treatment? Simple methods - PP Per-protocol • 1. Excludes patients who switch Assumptions: Switchers have same prognosis as non-switchers so selection bias not introduced • 2. Censor patients at time of switch Assumptions: Decision to switch not related to prognosis so censoring non-informative Simple methods - TTV Treatment as a time-varying covariate Time-to-event model adjusted for time-dependent treatment covariate: trt= 0, whilst receiving control treatment 1, whilst receiving experimental treatment Breaks randomisation balance and so subject to selection bias if switching related to prognosis Complex methods Rank Preserving Structural Failure Time Model • Attempt to estimate survival time lost/gained by exposure to experimental treatment • Relate the observed survival time, Ti, to the counterfactual survival time, Ui by 𝑈𝑖 = 𝑇𝑖0 + 𝑒 𝜑 𝑇𝑖1 Time on control treatment Acceleration factor Time on experimental treatment RPSFTM • For patients (always) treated with control treatment: Ti1=0 Ti=Ui • For patients (always) treated with experimental treatment Ti0=0 Ti=𝑒 −𝜑 Ui • Experimental treatment ‘multiplies’ survival time by 𝑒 −𝜑 relative to control treatment RPSFTM Randomisation Control patient Death Observed Counterfactual Death Treatment Observed patient Counterfactual Control patient who switches Expected survival time without active treatment – `shrunk’ by a factor of 𝑒 −𝜑 Death Observed Counterfactual Time RPSFTM Grid search for 𝜑: • Vary values of 𝜑 by a small amount between two plausible minimum and maximum values • Transform observed survival times using 𝑈𝑖 = 𝑇𝑖0 + 𝑒 𝜑 𝑇𝑖1 • Compare the counterfactual survival times between the two randomised groups (e.g., logrank test or Cox model) • Let 𝜑∗ be value of 𝜑 which maximises the p-value ∗ 𝜑 from the test, then acceleration factor is 𝑒 Assumptions • Randomisation based treatment effect estimator • Rank preserving: if patient i fails before patient j on treatment A, then i would fail before j on treatment B • Assumes the treatment effect is the same regardless of when patient starts to receive experimental treatment Complex methods Iterative parameter estimation algorithm • Extension of RPSFTM methods • Assume the same causal model relating actual and counterfactual survival times 𝑈𝑖 = 𝑇𝑖0 + 𝑒 𝜑 𝑇𝑖1 • Different estimation process for 𝜑 IPE • A parametric accelerated failure time model is fit to the observed survival times (e.g., Exponential, Weibull) • Initial estimate of acceleration factor 𝑒 𝜑 is obtained • This is used to create first counterfactual dataset, U1, using 1 𝑈 𝑖 = 𝑇1𝑖0 + 𝑒 𝜑 𝑇1𝑖1 IPE • Same parametric accelerated failure time model is fit to the counterfactual survival time • New estimate of 𝑒 𝜑 obtained • New counterfactual dataset created Until estimate of 𝑒 𝜑 converges (is within, say, 10-5 of the previous estimate) AF or HR? Note • strbee Stata program (Ian White) • ipe option • hr option • Final estimate of 𝑒 𝜑 , used to ‘correct’ observed survival times • Proportional hazards model used to estimate ‘corrected’ HR Application to VenUS IV Method Treatment effect form Estimate 95% CI P-value ITT HR 0.99 (0.79, 1.25) 0.96 PP_EXC HR 1.10 (0.86, 1.41) 0.43 PP_CENS HR 1.23 (0.98, 1.54) 0.08 TTV HR 1.20 (0.95, 1.50) 0.13 RPSFTM_log HR 0.92 (0.66, 1.28) 0.63 RPSFTM_cox HR 0.91 (0.69, 1.21) 0.53 IPE_exp HR 0.89 - - IPE_wei HR 0.88 - - Simulation • A simulation study suggested that the simple methods can significantly overestimate the true treatment effect, whilst the more complex methods of RPSFTM and IPE produce less biased results Conclusion • ITT analysis recommended as primary analysis • Consider a method to estimate the true effect of efficacy as secondary analysis, but not PP • Different methods can be used for continuous or categorical variables, e.g. CACE analysis Acknowledgements • York Trials Unit • VenUS IV trial team • Supervisor, Professor Mike Campbell (ScHARR, Sheffield) References • • • • • Ashby, R. L., et al. (2014). "Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (Venous leg Ulcer Study IV, VenUS IV): a randomised controlled trial." The Lancet 383(9920): 871-879. Robins, J. and A. Tsiatis (1991). "Correcting for non-compliance in randomized trials using rank preserving structural failure time models." Communications in Statistics-Theory and Methods 20(8): 2609 - 2631. White, I., et al. (1999). "Randomization-based methods for correcting for treatment changes: Examples from the Concorde trial." Statistics in Medicine 18(19): 2617 - 2634. White, I., et al. (2002). "strbee: Randomization-based efficacy estimator." The Stata Journal 2(Number 2): 140 - 150. Branson, M. and J. Whitehead (2002). "Estimating a treatment effect in survival studies in which patients switch treatment." Statistics in Medicine 21: 2449 - 2463.