Average Atomic Mass
advertisement

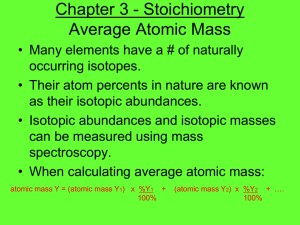
Chapter 3 - Stoichiometry Average Atomic Mass • Many elements have a # of naturally occurring isotopes. • Their atom percents in nature are known as their isotopic abundances. • Isotopic abundances and isotopic masses can be measured using mass spectroscopy. • When calculating average atomic mass: atomic mass Y = (atomic mass Y1) x %Y1 + 100% (atomic mass Y2) x %Y2 + …. 100% Determining Average Atomic Mass • Silver (Ag; Z = 47) has 46 known isotopes, but only two occur naturally, 107Ag and 109Ag. Given the following mass spectrometric data, calculate the atomic mass of Ag: • Atomic mass of Ag = 55.42 amu + 52.45 amu = 107.87 amu Avg. Atomic Mass Example Problem • Bromine consists of two isotopes: Bromine-79 (78.92 amu) and Bromine-81 (80.92 amu). What is the abundance of the heavier isotope? • 79.90 = 78.92 (100-x) + 80.92 (x) 100 or • 79.90 = .7892 (100-x) + .8092 (x) x = 49% of Br-81 100 The Mole • The mole represents 6.022 x 1023 items – Atoms, formula units, molecules • The MM, in grams/mole, is equal to the sum of the masses of the atoms in the formula. – i.e. MM of H2O = ? • Mole-gram conversions: m = MM x n • Mole-liters conversions: 22.4 L = 1 mole – This may only be used at STP Information Contained in the Chemical Formula of Glucose, C6H12O6 (MM = 180.16 g/mol) Mole Conversions - Example • Acetylsalicylic acid, C9H8O4, is the active ingredient in aspirin. a) What is the mass (g) of 0.509 mol of acetylsalicylic acid? b) What is the mass of H in this sample? c) How many moles of acetylsalicylic acid are in a onegram sample of aspirin that contains 91.6% by mass of acetylsalicylic acid? • a) 91.7 g C9H8O4 b) 4.10g H c) 5.08 x 10-3 mol Percent Composition • This allows us to determine the mass percents of the elements present in a compound. • Calculate the % composition of all elements in water: – Hydrogen - 11.19% – Oxygen - 88.81% % Composition Example • Metallic iron is most often extracted from hematite ore, which consists of iron (III) oxide mixed with impurities such as silicon dioxide. a) What are the mass percents of iron and oxygen in iron (III) oxide? b) How many grams of iron can be extracted from one kilogram of iron (III) oxide? c) How many metric tons of hematite ore, 66.4% iron (III) oxide, must be processed to produce one kilogram of iron? • (a)69.94% Fe, 30.06% O (b) 699.4g Fe (c)2.14 x 10-3 metric tons % Composition Example 2 • Hexachlorophene, a compound made up of atoms of carbon, hydrogen, chlorine, and oxygen. Combustion of a 1.000gsample yields 1.407g of carbon dioxide, 0.134 g of water, & 0.523 g of chlorine gas. What are the mass percents of all the elements that make up hexachlorophene? – 38.40% C, 1.50% H, 52.3% Cl, 7.80% O Empirical Formula • The empirical formula of a compound is the simplest ratio possible for 2 or more elements. – CH4 C2H6 – CH5N C2H10N2 – To solve for the empirical formula you are generally given the %composition or masses of the elements in the compound from combustion analysis. Combustion Analysis Empirical Formula - Example • Determine the empirical formula for a compound that gives the following percentages on analysis: • 71.65% Cl, 24.27% C, 4.07% H • ClCH2 Steps to an Empirical Formula Molecular Formula • This is basically multiples of the empirical. • You need to know the molar mass of the sample and its empirical formula to find. • In the last example we determined the empirical formula to be ClCH2, what if you were told this sample had a molar mass of 98.96 g/mol, how would you find the molecular formula? • Cl2C2H4 Empirical & Molecular Formula Example • A white powder is analyzed and found to contain 43.64% phosphorous and 56.36% oxygen by mass. The compound has a molar mass of 283.88 g/mol. What are the compounds empirical and molecular formulas? • Empirical = P2O5 Molecular = P4O10 Chemical Equations • Reactants & products in a chemical equations represent what is happening in a chemical reaction. • Coefficients are used to balance these equations. • States of matter are not required on the AP Chemistry exam however any aqueous solution must be written as ions in aqueous solution. Balancing Chemical Equations » (a) A characteristic reaction of Group 1A(1) elements: chunks of sodium react violently with water to form hydrogen gas and sodium hydroxide solution. » (b) The destruction of marble statuary by acid rain: aqueous nitric acid reacts with calcium carbonate to form carbon dioxide, water, and aqueous calcium nitrate. » (c) Halogen compounds exchanging bonding partners: phosphorus trifluoride is prepared by the reaction of phosphorus trichloride and hydrogen fluoride; hydrogen chloride is the other product. The reaction involves gases only. » (d) Explosive decomposition of dynamite: liquid nitroglycerine (C3H5N3O9) explodes to produce a mixture of gases—carbon dioxide, water vapor, nitrogen, and oxygen Stoichiometric Calculations • The coefficients in a balanced equation give you a molar ratio of the reactants and products. This ratio is used to convert from one substance to another in a balanced chemical equation. • Solid lithium hydroxide is use in space vehicles to remove exhaled carbon dioxide from the living environment by forming solid lithium carbonate and liquid water. What mass of gaseous carbon dioxide can be absorbed by 1.00 kg of lithium hydroxide? – 9.20 x 102 g of CO2 Stoichiometric Calculation - Example • Baking soda is often used as an antacid. It neutralizes excess HCl acid secreted by the stomach: NaHCO3(s) + HCl(aq) NaCl(aq) + H2O(l) + CO2(aq) Milk of Magnesia, which is an aqueous suspension of magnesium hydroxide, is also used as an antacid: Mg(OH)2(s) + 2HCl(aq) 2H2O(l) + MgCl2(aq) Which is the more effective antacid per gram, the baking soda or milk of magnesia? -Baking soda neutralizes 1.19 x10-2 mol HCl while milk of magnesia neutralizes 3.42 x10-2 mol HCl. So milk of magnesia is more effective. The Limiting Reactant The Limiting Reactant • This reactant limits the amount of product produced because it runs out first: – Nitrogen gas can be prepared by passing gaseous ammonia over solid copper (II) oxide at high temperatures. The other products of the reaction are solid copper and water vapor. If a sample containing 18.1 g of ammonia is reacted with 90.4 g of copper (II) oxide, how many grams of nitrogen will be formed? • 10.6 g of N2 Percent Yield • The actual yield of product is generally given as a percentage calculated from the following formula: % Yield = actual yield x 100 theoretical yield % Yield Example • Silicon carbide (SiC) is an important ceramic material that is made by allowing sand (silicon dioxide, SiO2) to react with powdered carbon at high temperature. Carbon monoxide is also formed. When 100.0 kg of sand is processed, 51.4 kg of SiC is recovered. What is the percent yield? Sources • (Silberberg, Martin S.. Chemistry: The Molecular Nature of Matter and Change, 5th Edition. McGraw-Hill, 012009. 3.7.5).