5.4.2 Organic nitrogen compounds: amines, amides, amino acids
advertisement

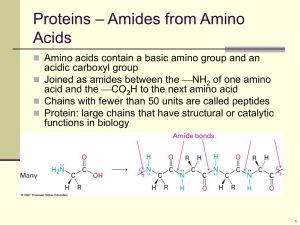
5.4.2 Organic nitrogen compounds: amines, amides, amino acids and proteins Benzene Reaction summary http://en.labs.wikimedia.org/wiki/ A-level_Chemistry/OCR_%28 Salters%29/Reactions_of_arenes • Burning Benzene – Smokey flame • http://www.youtube.com/watch?v=XhRHk4 zLR8A Methoxybenzene and methylbenzene (toluene) • Also undergo E+ subn. Do so more readily than benzene. Phenol, C6H5OH, (carbolic acid ) Phenol, C6H5OH • Early anesthetic (but burned the skin) • Poorly soluble (8.3 g dissolving in 100 mL ) • Does NOT behave like an aliphatic alcohol - is acidic, pKa = 10.0 (ethanol=15.9, ethanoic acid=4.75) • Although acidic, unlike –COOH, phenol NOT will react with carbonates and hydrogencarbonates to form CO2 • Only esterifies with acid chloride (i.e. not with a carbox. Acid, RCOOH) …RING MUCH MORE REACTIVE THAN BENZENE Solubility of phenol and phenoxide anion. Phenol in water (org solvent at bottom) > NaOH added > acidified http://www.sciencephoto.com/image/4210/530wm/A5000274- Phenol_reactions-SPL.jpg Phenol with Br2(aq) c.f. benzene! Phenol with cold dil HNO3 c.f. benzene! • http://upload.wikimedia.org/wikipedia/commons/5/5c/EAS-ortho-para-meta.png E+ Subn in phenol • Many (Kekule)resonance structures http://chem-guide.blogspot.com/2010/04/electrophilic-substitution-of-phenol.html Rxns • of benzene http://clem.mscd.edu/~wiederm/oc2chp/oc2chpphenols/phenolrxnsummary.gif Other Reactions (N.O.S.) - Demonstrate increased reactivity of the ring Aspirin (synthesis #1) • http://www.ch.ic.ac.uk/rzepa/mim/drugs/html/aspirin_text.htm Aspirin (synthesis #2) • http://www.ch.ic.ac.uk/rzepa/mim/drugs/html/aspirin_text.htm Amines, amides and amino acids • Aliphatic amines, & aromatic amines ethylamine phenylamine a. give examples of: i. molecules that contain amine and amide functional groups ii. amino acids • b. describe and carry out, where appropriate (using butylamine and phenylamine), reactions to investigate the typical behaviour of primary amines. This will be limited to: • i. characteristic smell • ii. miscibility with water as a result of hydrogen bonding and the alkaline nature of the resulting solution • iii. formation of salts • iv. complex ion formation with copper(II) ions • v. treatment with ethanoyl chloride and halogenoalkanes, eg making paracetamol Making paracetamol #1 • para-acetylaminophenol http://www.ch.ic.ac.uk/rzepa/mim/drugs/html/paracet_text.htm (good short notes on overdose problem) Making paracetamol #2 • http://en.wikipedia.org/wiki/Paracetamol Reduction of Nitrobenzene to phenylamime (aniline) Diazo formation • 1o, 2o and 3o aromatic based amines. Video 1o amine undergoing decomposition T>10oC Methyl orange via diazo coupling http://www.science.cmu.ac.th/educate/ couresware_chem/203204/Arputtinan6.htm The colours of Methyl Orange < A diazo dye • Other Azocompounds (salters chem) Polymers • Video of polymer – ‘bubblegum’ a.k.a. Plastics, rubber, food colouring, sucrose and dextrose – yum huh? Nylons… • Video 1 – intro to polymers • Video 2 – nylon – brave? no glasses guy • Video 3 – specific chemicals used nylon • Product name : SAP-Super Absorbent Polymer • Chemical name : Sodium polyacrylate • Monomer Molecular formula : C3H4O2 monomer Many COOH (or COONa) groups allow for widespread large amount of interaction with water molecules ? = addition polymer repeating unit of sodium poly(acrylate) Super Absorbent Polymers for Diaper http://www.made-in-china.com Video Other water interacting polymers • Propeneamide Dissolving Plastics Poly(ethanol) Propeneamide •Webpage: DissolvingPolymers.mht ? = addition polymer repeating unit of poly(propeneamide) Dun WORi, POLYURETHANES ARE NOT ON THE SYLLABUS !!! Polyurethane formation (isocyanate with polyol) PU video http://ec.gc.ca/substances/nsb/images/Polyurethane_S1.gif Condensation polymers • Terylene is used to make fishing nets clothes (quick-dry, non-iron) cassette and video tapes Condensation polymers: There are two main types of condensation polymer made from carboxylic acid derivatives: polyesters and polyamides. i) Polyesters: A well-known polyester, Terylene, is made by heating ethane-1,2-diol with dimethylbenzene-1,4-dicarboxylate (dimethyl terepthalate): The dimethlybenzene-1,4-dicarboxylate is obtained via the following reaction pathway: The manufacture of ethane-1,2-diol has already been described in section 26.4.2. Or simpler rxn (more edexcel friendly) not using the diester as previously, but the dicarbox acid… Kevlar Poly(N-substituted amide) Kevlar again. Synthesis of Kevlar Should be familiar with monomers and polymer requirements now. Poly(N-substituted) amide – similar to Nylon Amino acids. • Naturally occurring ones are L acids (laevorotatory) • Strecker synthesis give L and R (next slide) T h e S tre c k e r s yn th e s is . - S yn th e s is o f a m in o a c id s (1) (2) O R - (1) . O. .. C H H (3) (2) (1) R H C N H H O w a te r (2) + N H3 H H . O. H R C .. N H H H R C .. N H H a n im in e (1) H H (2) R C N H H T h e n itro g e n ve rs io n o f a c a rb o n yl. C = N c a n re p la c e C = O (re c a ll 2 ,4 -D N P re a c tio n !!) (2) .. CN CN R O C .. . N. H H CN R (1) T h e im in e C .. N H H H ( +O H - ions) T he N equivalent of the alpha-hydroxy nitriles. A n alpha-am ine nitrile Lik e C = O 's, C = N 's are susceptable to nucleophilic attack - addition - by - C N A cid hydrolysis of niriles. OH O C R *C .. N H H H A n alpha-am ino acid The amino acids vary in their side chains (indicated in blue in the diagram). The eight amino acids in the orange area are nonpolar and hydrophobic. The other amino acids are polar and hydrophilic ("water loving"). The two amino acids in the magenta box are acidic ("carboxy" group in the side chain). The three amino acids in the light blue box are basic ("amine" group in the side chain). Amino acids – Ninhydrin rxn and test Ninhydrin rxn with amino acids. Procedure Add about 2 mg of the sample to 1 mL of a solution of 0.2 g of ninhydrin (1,2,3indanetrione monohydrate) in 50 mL of water. The test mixture is heated to boiling for 15-20 sec; This reaction is important not only because it is a qualitative test, but also because it is the source of the absorbing material that can be measured quantitatively by an automatic amino acid analyzer. This color reaction is also used to detect the presence and position of amino acids after paper chromatographic separation. Positive Test A blue to blue-violet color is given by a-amino acids and constitutes a positive test. Other colors (yellow, orange, red) are negative. Complications Proline, hydroxyproline, and 2-, 3-, and 4-aminobenzoic acids fail to give a blue color but produce a yellow color instead. Ammonium salts give a positive test. Some amines, such as aniline, yield orange to red colors, which is a negative test. Amino acids – analysis with ninhydrin chromatography Protein synthesis (biological) • Video (real time) • Video 2 • Salters Organic Toolbox • Salters Organic Reactions.mht