MATERI 3. RT-Biogeokimia
advertisement

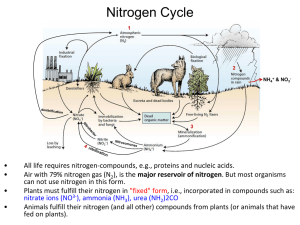
MIKROORGANISME DALAM SIKLUS BIOGEOKIMIA Oleh: Dr. Ratu Safitri, MS Laboratorium Mikrobiologi. Jurusan Biologi F-MIPA Universitas Padjadjaran LINGKUP MATERI: Ikhtisar Siklus Biogeokimia : - Siklus N dan Reaksi dalam siklus - Siklus O - Siklus P - Siklus C 2 DASAR DARI KONSEP SIKLUS BIOGEOKIMIA Semua materi siklus tidak diciptakan atau dihancurkan Sehubungan karena bumi adalah suatu sistem tertutup, maka semua hal yang berada di dalamya akan dalam suatu siklus. Siklus Biogeokimia: perbahan atau sklus materi dalam suatu sistem lingkungan. 3 JENIS MATERI YANG BEREDAR Element kimia (carbon, nitrogen, oxygen, sulfur , Phosphor) atau molekule air . Makronutient : diperlukan pertukaran dalam jumlah yang besar, misal : potassium , calcium , iron , magnesium Mikronutrien beredar dalam jumlah yang sangat kecil, misalnya: boron (tanaman hijau) copper (untuk aktifitas ensim) molybdenum (nitrogen-fixing bacteria) 4 - EARTH’S ECOSYSTEMS ARE MAINTAINED BY A CONSTANT INFLUX OF ENERGY Transformation Loss of Energy Solar Energy Autotroph Herbivore Respiratory Loss Carnivore 5 BIOGEOCHEMICAL CYCLES Cycling of chemical elements between living and nonliving portions of the earth’s ecosystems Decomposition Respiration Excretion Biotic Uptake Abiotic BIOGEOCHEMICAL CYCLE: Siklus utama yang akan dibahas: Siklus nitrogen Siklus oxygen Siklus phosphorus Siklus carbon Sirkulasi molekul kimia dalam siklus biogeokimia dan interaksinya dalam siklus adlah sangat penting untuk memelihara ekosistem terestrial, air tawar, dan ekosistem laut. Perubahan iklim global, temperatur, hujan, dann kestabilan ekosistem sangat tergantung pada siklus biogeokimia. 7 Siklus N 8 NITROGEN BEREDAR DI TANAH -Komposisi N udara: 80% - Nitrogen beredar dalam peredaran : (a). Bakteri dalam tanah akan merubah nitrat menjadi gas ke udara (denitrifikasi) (b) Dengan adanya cahaya, sejumlah nitrogen dioksidasi dan bergabung dengan air membentuk asam dan akan jatuh dalam bentuk hujan. •Tanaman akan mengambil nitrat dan mengubahnya menjadi bahan protein yang akan diantarkan oleh karnivora dan herbivora dalam rantai makanan. •Ketika organisma mengelurakan limbah, nitrogen akan dikembalikan ke lingkunga. Ketika biota mati, akan didekomposisi dan dikonversikan menajdi 9 amoniak. 10 11 Surface water Oxidized layer Reduced soil layer Low [NH4] Biodegradation Slow Diffusion C/N <20 C/N >20 [NH4] HIGH 12 Surface water Oxidized layer Reduced soil layer nitrification Low [NH4] [NO3] high Slow Diffusion [NH4] HIGH 13 N2 Surface water Oxidized layer Reduced soil layer [NO3] high Leaching [NO3] Low Denitrification 14 Nitrogen Fixation 15 Nodules on plant roots REAKSI-REAKSI DALAM SIKLUS NITROGEN SUMBER N Lightning Inorganic fertilizers Nitrogen Fixation Animal Residues Crop residues Organic fertilizers 17 FORMS OF NITROGEN Urea CO(NH2)2 Ammonia NH3 (gaseous) Ammonium NH4 Nitrate NO3 Nitrite NO2 Atmospheric Dinitrogen N2 Organic N ROLES OF NITROGEN Plants and bacteria use nitrogen in the form of NH4+ or NO3 It serves as an electron acceptor in anaerobic environment Nitrogen is often the most limiting nutrient in soil and water. 18 GLOBAL NITROGEN RESERVOIRS Nitrogen Reservoir Atmosphere Metric tons nitrogen 3.9*1015 Actively cycled Ocean soluble salts Biomass 6.9*1011 5.2*108 Yes Yes Land organic matter Biota 1.1*1011 2.5*1010 Slow Yes No 19 NITROGEN IS A KEY ELEMENT FOR amino acids nucleic acids (purine, pyrimidine) cell wall components of bacteria (NAM). 20 NITROGEN CYCLES Ammonification/mineralization Immobilization Nitrogen Fixation Nitrification Denitrification 21 N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 22 AMMONIFICATION OR MINERALIZATION N2 N2O NH4 NO2 R-NH2 NO NO2 NO3 23 MINERALIZATION OR AMMONIFICATION Decomposers: earthworms, termites, slugs, snails, bacteria, and fungi Uses extracellular enzymes initiate degradation of plant polymers Microorganisms uses: Proteases, lysozymes, nucleases to degrade nitrogen containing molecules Plants die or bacterial cells lyse release of organic nitrogen Organic nitrogen is converted to inorganic nitrogen (NH3) When pH<7.5, converted rapidly to NH4 Example: Urea NH3 + 2 CO2 24 IMMOBILIZATION The opposite of mineralization Happens when nitrogen is limiting in the environment Nitrogen limitation is governed by C/N ratio C/N typical for soil microbial biomass is 20 C/N < 20 Mineralization C/N > 20 Immobilization 25 NITROGEN FIXATION Energy intensive process : N2 + 8H+ + 8e- + 16 ATP = 2NH3 + H2 + 16ADP + 16 Pi Performed only by selected bacteria and actinomycetes Performed in nitrogen fixing crops (ex: soybeans) N 2 N2O NH4 NO2 R-NH2 NO 26 NO2 NO3 MICROORGANISMS FIXING Azobacter Beijerinckia Azospirillum Clostridium Cyanobacteria Anabaena Require the enzyme nitrogenase Inhibited by oxygen Inhibited by ammonia (end product) Microcystis 27 RATES OF NITROGEN FIXATION N2 fixing system Rhizobium-legume Nitrogen Fixation (kg N/hect/year) 200-300 Cyanobacteria- moss 30-40 Rhizosphere associations Free- living 2-25 1-2 28 BACTERIAL FIXATION Occurs mostly in salt marshes Is absent from low pH peat of northern bogs Cyanobacteria found in waterlogged soils 29 NITRIFICATION Two step reactions that occur together : 1rst step catalyzed by Nitrosomonas 2 NH4+ + 3 O2 2 NO2- +2 H2O+ 4 H+ N2 2nd step catalyzed by Nitrobacter 2 NO2- + O2 2 NO3- Optimal pH is between 6.6-8.0 If pH < 6.0 rate is slowed If pH < 4.5 reaction is inhibited N2 O N H4 NO 2 RNH2 N O NO2 NO 30 3 DENITRIFIKASI N2 Removes a limiting nutrient from the environment 4NO3 + C6H12O6 2N2 + 6 H 20 Inhibited by O2 Not inhibited by ammonia Microbial reaction Nitrate is the terminal electron acceptor N2 O NH NO2 4 R-NH2 NO NO2 NO3 31 Interactive Nitrogen Cycle Atmosphe re N2 Fixation Industria l Process es Plant and Animal Residues Lightning , Rainfall Fertilizer Pla nt Los s Volatilizati on R-NH2 + Energy + CO2 Organic Matter N2, N2O, NO R-NH2 + H2O Plant/Micro bial Sink NO3Pool R-OH + Energy + 2NH3 Soil exchange sites 2NH4+ + 2OH- 32 Leachin g 2NO2- + H2O + 4H+ Back to Intro Page Back to Intro Page GLOBAL WARMING ATMOSPHERE N2O NO N2 PLANT LOSS INDUSTRIAL FIXATION N2 FIXATION SYMBIOTIC NON-SYMBIOTIC MESQUITE RHIZOBIUM ALFALFA SOYBEAN BLUE-GREEN ALGAE AZOTOBACTER CLOSTRIDIUM LIGHTNING, RAINFALL PLANT AND ANIMAL RESIDUES HABER BOSCH (1200°C, 500 atm) 3H2 + N2 2NH3 MATERIALS WITH N CONTENT > 1.5% (COW MANURE) FERTILIZATION MATERIALS WITH N CONTENT < 1.5% (WHEAT STRAW) AMINO ACIDS NH3 AMMONIA VOLATILIZATION AMINIZATION ORGANIC MATTER HETEROTROPHIC R-NH2 + ENERGY + CO2 BACTERIA (pH>6.0) FUNGI (pH<6.0) IMMOBILIZATION Pseudomonas, Bacillus, Thiobacillus Denitrificans, and T. thioparus R-OH + ENERGY + 2NH3 N2O2MINERALIZATION + NITRIFICATION MICROBIAL/PLANT SINK 2NH4+ + 2OHFIXED ON EXCHANGE SITES NO2- OXIDATION STATES NH3 AMMONIA -3 NH4+ AMMONIUM -3 N2 DIATOMIC N 0 N2O NITROUS OXIDE 1 NO NITRIC OXIDE 2 NO2- NITRITE 3 NO3 NITRATE 5 pH>7.0 R-NH2 + H2O AMMONIFICATION NH2OH NO3POOL DENITRIFICATION LEACHING TEMP 50°F LEACHING NITRIFICATION 2NO2- + H2O + 4H+ Nitrobacter LEACHING VOLATILIZATION NITRIFICATION LEACHING + O2 Joanne LaRuffa Robert Mullen Wade Thomason Susan Mullins Shannon Taylor Heather Lees LEACHING pH 7.0 +O2 Department of Plant and Soil Sciences Oklahoma State University ADDITIONS LOSSES 33 OXIDATION REACTIONS REDUCTION REACTIONS SIKLUS OKSIGEN 34 Tanaman menggunakan energi matahari untuk mengkonversikan karbondioksida dan air menjadi karbohidrat dan oksigen melalui fotositesis. 6CO2 + 6H2O + energy → C6H12O6 + 6O2 Organisma fotosintetik berperan dalam siklus oksigen termasuk tanaman , phytoplankton di laut. Biota laut cyanobacteria Prochlorococcus ditemukan tahun 1986. Hwan membentuk setengah siklus dari oksigen yang digunakan untuk memecah karbohidrat menjadi energi dalam proses respirasi. O2 + carbohydrates → CO2 + H2O + energy 35 SIKLUS P 36 SIKLUS P DI LAUTAN 37 SIKLUS P DI DALAM TANAH 38 The phosphorus cycle describes the movement of phosphorus through the lithosphere, hydrosphere, and biosphere. The atmosphere does not play a significant role, because phosphorus and phosphorus-based compounds are usually solids at the typical ranges of temperature and pressure found on Earth. Phosphorus normally occurs in nature as part of a phosphate ion, consisting of a phosphorus atom and some number of oxygen atoms, the most abundant form (called orthophosphate) having four oxygens: PO43-. Most phosphates are found as salts in ocean sediments or in rocks. Over time, geologic processes can bring ocean sediments to land, and weathering will carry terrestrial phosphates back to the ocean. 39 Plants absorb phosphates from the soil and phosphate enters the food chain. After death, the animal or plant decays, and the phosphates are returned to the soil. Runoff may carry them back to the ocean or they may be reincorporated into rock. The primary biological importance of phosphates is as a component of nucleotides, which serve as energy storage within cells (ATP) or when linked together, form the nucleic acids DNA and RNA. Phosphorus is also found in bones, and in phospholipids (found in all biological membranes). Phosphates move quickly through plants and animals; however, the processes that move them through the soil or ocean are very slow, making the phosphorus cycle overall one of the slowest biogeochemical cycles. 40 Siklus Karbon 41 Usually thought of as four major reservoirs of carbon (the atmosphere, the terrestrial biosphere - which includes freshwater systems and non-living organic material, such as soil carbon -, the oceans with dissolved inorganic carbon and living and non-living marine biota, and the sediments which includes fossil fuels) interconnected by pathways of exchange. The exchanges between reservoirs, occur because of various chemical, physical, geological, and biological processes. The ocean contains the largest active pool of carbon near the surface of the Earth, but the deep ocean part of this pool does not rapidly exchange with the atmosphere. 42 The global carbon budget is the balance of the exchanges (incomes and losses) of carbon between the carbon reservoirs or between one specific loop (e.g., atmosphere - biosphere) of the carbon cycle. IN THE OCEAN: The seas contain around 36000 gigatonnes of carbon, mostly in the form of bicarbonate ion. Inorganic carbon, that is carbon compounds with no carboncarbon or carbon-hydrogen bonds, is important in its reactions within water. This carbon exchange becomes important in controlling pH in the ocean and can also vary as a source or sink for carbon. 43 Carbon is readily exchanged between the atmosphere and ocean. In regions of oceanic upwelling, carbon is released to the atmosphere. Conversely, regions of downwelling transfer carbon (CO2) from the atmosphere to the ocean. When CO2 enters the ocean, carbonic acid is formed: CO2 + H2O ⇌ H2CO3 This reaction has a forward and reverse rate, that is it achieves a chemical equilibrium. Another reaction important in controlling oceanic pH levels is the release of hydrogen ions and bicarbonate. This reaction controls large changes in pH: H2CO3 ⇌ H+ + HCO3− 44