Basic Chemistry and Cell Structure
advertisement

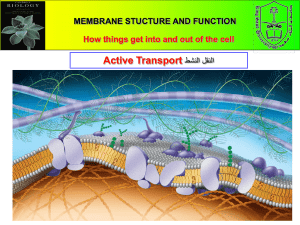
Biol 155 Human Physiology Instructor: Dr. Robert Harris Office: 1354 Biological Sciences Phone: 822-5709 Email: harris@zoology.ubc.ca Course requirements: Texts: Fundamentals of Anatomy and Physiology 6th ed. Frederic H. Martini Anatomy & Physiology Coloring Workbook, 7th ed. Elaine Marieb Read course synopsis!!! Failure to read it, or failure to listen to what I say does not constitute an excuse Lecture Notes and synopsis are posted at: http://www.zoology.ubc.ca/~biomania/biol153/ lecture/main01.htm Mark Breakdown Biol 153: The lecture mark is based on: Lecture: Lab: Course Total: One mid-term exam in each term: Winter exam: Final exam: Total: 60% 40% 100% 20% 20% 20% 60% (10% each) Mark Breakdown cont. Biol 155: marks will be based solely on the lecture exams, which will be weighted as follows: One mid-term exam in each term: 30% (15% each) Anatomy colouring book 5% Winter exam: 30% Final exam: 35% Total: 100% Atomic structure and elements An element is a substance that retains its chemical and physical characteristics even when it is broken down into its smallest units. The smallest practical unit, for our purposes is the atom. The chemical characteristics are determined by the number of protons These are the three forms of hydrogen All three have one electron All three have one proton Electron orbits Number of electrons generally equals number of protons. There are specific orbits (or shells), that contain a specific maximum number of electrons Charged atoms Atoms are most stable when there are 8 electrons in the outermost shell. In order for the outermost shell to be filled, atoms will either take in or give off electrons. When this happens there is a change in net charge. Charged atoms (ions) can be electrically attracted (opposite charges attract) This is known as ionic bonding Ionic bonds are fairly weak Covalent bonds Another way atoms can fill their outer shell is to share electrons with another atom The electrons orbit around BOTH nuclei This is known as a covalent bond Covalent bonds are much stronger than ionic bonds Molecular dipoles When covalent bonds are formed, the electrons may not be shared equally between the atoms This unequal sharing can result in an uneven distribution of electrical charges on the molecule This is known as a partial charge, or a dipole Hydrogen bonding Water molecules interact with each other electrically The partial negative charge around the oxygen is attracted to the partial positive charge around the hydrogen These very weak electrical attractions are called hydrogen bonds Ions in aqueous solution Water molecules can form hydrogen bonds with ions Ions in solution have a layer of tightly bound water molecules around them This layer of water molecules is known as the hydration sphere Water can form hydrogen bonds with uncharged molecules as well (providing there is a partial charge) pH is the negative log (the small p) of the hydrogen concentration (the large H) In pure water, some of the H2O molecules will dissociate into H+ and OHThe H+ concentration in pure water is 0.1 mM, or 1x10-7 moles/L (hence pH 7) Molecular Representations There are several ways or representing molecular structures Here are three representations of glucose Linear model Structural model Space-filling model Synthetic and Lytic Reactions Smaller organic molecules can be linked together Often this involves the production of H2O Larger organic molecules can be broken down into subunits This often consumes H2O, hence the term “Hydrolysis” Energetics of chemical reactions In order for chemicals to react, they must first overcome an energy barrier This is known as the activation energy Some bonds are easily reorganized, resulting in a lower activation energy Enzyme catalyzed reactions Enzyme has binding sites for the reactants The active region will attack the bonds in the precursors Once bonds have been reorganized, product is released Polymers in organic systems A polymer is a chain made up of repeating subunits Useful compounds are often stored in the form of a polymer For example, glycogen is a branched polymer of glucose Glycogen molecules can have different numbers of glucose subunits Proteins are also polymers Fatty acids and lipids Phospholipids in aqueous solutions Phospholipids and glycolipids are amphipathic Meaning they have a hydrophillic region and a hydrophobic region When they are in solution, they form micelles Structure of Amino Acids All amino acids have the same basic structure A carboxylic acid side An amino group side A side group on the central carbon The side group is referred to as the Rgroup Primary protein structure Secondary protein structure The chain of amino acids can form folds and coils in different regions, depending on the amino acid sequence Tertiary protein structure The tertiary structure of a protein is the 3D shape of a single subunit. This is a combination of all the folds, coils and sheets. This is usually dictated by hydrophobic and hydrophilic interactions with water Tertiary and Quaternary protein structure The quaternary structure of a protein is the interactions between the different subunits If a protein is only composed of a single subunit, there is no quaternary structure DNA and RNA structure Adenosine triphosphate (ATP) Adenosine backbone Three phosphate groups attached in a chain Last two have high energy bonds Characteristics of a lipid bilayer: At normal temperatures, a lipid bilayer is liquid. This means that the phospho- and glycolipids which make it up can move freely, within the bilayer. Because of the hydrophobic layer in the centre, a bilayer is impermeable to water. Because of the hydrophilic and hydrophobic interactions, a bilayer is structurally quite strong. Membrane fluidity Membrane proteins: Diffusion Effect of osmotic concentration on cells Cell membranes are semipermeable, and thus subject to osmotic forces. Animal cell membranes are flexible, and allow for inflation and deflation depending on the movement of water Transport of solutes through cell membranes Cell membranes are made up of phospholipids arranged in a bilayer. The centre of the bilayer is hydrophobic, which means that hydrophilic molecules cant penetrate. Hydrophobic and lipid-soluble molecules can penetrate cell membranes. In order for hydrophilic molecules to be taken up, a transport mechanism is needed. These transport mechanisms are integral membrane proteins. Ion channels Ions are fairly small molecules. Specialized proteins in the membrane form aqueous pores, which allow ions through. The driving force is the chemical gradient These pores can be quite selective. Most of these pores are regulated Example: CFTR Facilitated diffusion Molecules that are slightly larger need more help in getting into or out of cells. Rather than a pore, molecules are actually bound to carrier protein, which translocates molecule. Driving force is still the chemical gradient Active transport In order to move ions against a concentration gradient, energy must be used. Energy is supplied by the hydrolysis of the terminal high-energy bond of ATP. Example: Na-K-ATPase Active secondary transport ATPases only pump ions, nothing bigger. Larger molecules are transported by coupling them to movement of an ion down its concentration gradient. Ions can also be transported in this way. Example: Na-coupled glucose uptake. Membrane transport and cycling Molecules can bind to cell surface receptors and then be internalized. This same mechanism can be used to recycle membrane. Phagocytosis Phagocytosis also involves membrane invagination. This process does not involve clathrin. Pseudopods extend around a particle, forming a phagosome. Phagosome will fuse with a lysosome, containing digestive enzymes. There are smaller transport mechanisms in the wall of the secondary lysosome. Cellular organelles Most intracellular organelles are membranebound. Since membranes are barriers to diffusion of aqueous solutes, they allow for partitioning of cellular components Such partitioning allows for the generation of gradients and/or the segregation of specific compounds inside the cell, a process that is essential for life. Endoplasmic reticulum The endoplasmic reticulum consists of a series of interconnected membrane-bound tubes and lamina that are all continuous. It is essential in the production of membrane proteins. It also serves as a Ca2+ storage organelle. Ribosomes Ribosomes are enzymes made up of two subunits. Ribosomes are the enzyme that synthesize proteins, based on an mRNA template Some ribosomes are attached to the ER and some a free in the cytoplasm. Translation Golgi apparatus The Golgi apparatus is a contiguous system of lamellae and cisternae. It is responsible for post-translation modifications of proteins, formation of secretory vesicles and membrane formation and trafficking. Membrane flow Transport vesicles bud off the ER and are transported to the forming face of the Golgi. Membrane-bound proteins and secretory proteins then move through the Golgi, where they are modified, usually by glycosylation. The proteins and membranes are then packaged into specific vesicles, which are targeted. Mitochondria Mitochondria actually have two membranes, separated by a small space. Mitochondria also have their own DNA. Mitochondria are essential for oxidative phosphorylation. Nucleus Nucleus also has two membranes. Nucleus protects the DNA and maintains a specific environment for the DNA. Nuclear pores allow for transport into and out of nucleus. Library tutorial http://toby.library.ubc.ca/ereserve/er-coursepage. cfm?id=1416 For the Biol 153 students: Lee Ann Bryant (from the library) will be here at the end of the lecture to give a short talk about the library assignment. Cell cycle DNA condensation