Unit 15 Revision
advertisement
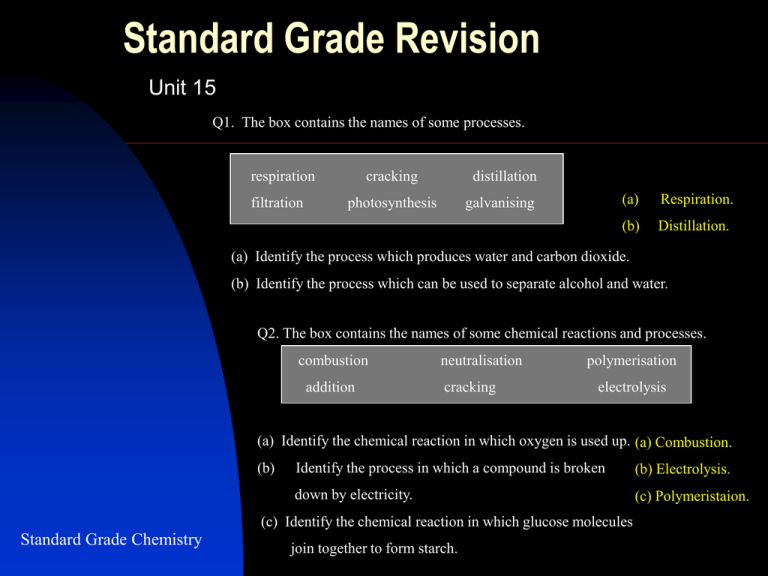
Standard Grade Revision Unit 15 Q1. The box contains the names of some processes. respiration filtration cracking distillation photosynthesis galvanising (a) Respiration. (b) Distillation. (a) Identify the process which produces water and carbon dioxide. (b) Identify the process which can be used to separate alcohol and water. Q2. The box contains the names of some chemical reactions and processes. combustion addition neutralisation cracking polymerisation electrolysis (a) Identify the chemical reaction in which oxygen is used up. (a) Combustion. (b) Identify the process in which a compound is broken (b) Electrolysis. down by electricity. (c) Polymeristaion. (c) Identify the chemical reaction in which glucose molecules Standard Grade Chemistry join together to form starch. Q3. Starch and glucose are carbohydrates. (a) Which chemical would you use to test for starch? (b) What is the chemical name for the alcohol produced by the fermentation of glucose? (c) The percentage of alcohol in wine depends on the temperature of the fermentation process. Some results are shown on the graph. (i) Describe how the temperature of fermentation affects the % alcohol produced? (ii) Use the graph to estimate the % alcohol when the temperature is 37oC (a) Iodine (turns blue black). (b) Ethanol. (c) (i) As the temperature increases the percentage of alcohol decreases. Standard Grade Chemistry (ii) 12.5% Unit 15 Revision Q4. The bar chart shows the boiling points of some alcohols. (a) Describe the relationship between the number of carbon atoms and the boiling point. Standard Grade Chemistry (b) Predict the boiling point of the alcohol with five carbon atoms per molecule. (c) Ethanol is the name of the alcohol with two carbon atoms per molecule. What would be the name of the alcohol with one carbon atom per molecule? (a) As the number of carbon atoms in the molecule increases the boiling point increases. (b) 130oC (c) Methanol. Unit 15 Revision. Q5. Alcoholic drinks contain different percentages of alcohol. Beers contain 5% but some ciders are stronger at 7.5%. Red wine contains 12%, but fortified wines contain 18%. Whisky is much stronger at 40% alcohol. (a) Present the above information in a table with suitable headings. (b) All alcoholic drinks are made using the same process: glucose alcohol + carbon dioxide (i) What type of substance, found in yeast, acts as a catalyst for the reaction? (ii) What is the correct chemical name for the alcohol found in alcoholic drinks? (iii) State a test for carbon dioxide. (a) Standard Grade Chemistry (b) Alcoholic drink Percentage Alcohol / % Beers 5 Ciders 7.5 Red wine 12 Fortified wine 18 Whisky 40 (i) Enzyme (zymase) (ii) Ethanol (iii) Turns lime water chalky (or milky) Unit 15 Revision. Q6. The equations represent chemical reactions involving carbohydrates. A carbon dioxide + water B glucose C starch + water D glucose E glucose + oxygen glucose + oxygen starch + water glucose ethanol + carbon dioxide carbon dioxide + water (a) Identify the reaction which is catalysed by enzymes in yeast. (a) D (b) Identify the hydrolysis reaction. (b) C (c) Identify the reaction which takes place in animals during respiration. (c) E Q7. Various reagents can be used to identify substances Standard Grade Chemistry A Benedict’s solution B bromine solution C ferroxyl indicator D iodine solution E lime water a) Identify the reagent used to test for starch. b) Identify the reagent used to test for glucose (a) D (b) A Unit 15 Revision. Q8. Glucose, sucrose and starch are carbohydrates. Identify the two correct statements. A Glucose molecules join together with a loss of water. B Starch is a polymer made from sucrose molecules. C Sucrose turns warm Benedict’s solution orange. D Glucose is an isomer of sucrose. E Starch dissolves easily in water. F Sucrose can be hydrolysed. A and F Q9. The equation shows the reaction in which glucose is converted into ethanol (alcohol). C6H12O6 2C2H5OH + 2CO2 (a) What name is given to this process? (b) Calculate the mass of ethanol that is obtained when 60 kg of glucose is totally converted into alcohol. Standard Grade Chemistry (a) Fermentation (b) 32kg Unit 15 Revision. Q. Methanol can take part in many chemical reactions. (a) (i) Compound X has the molecular formula CH2O. (a)(i) H Draw the full structural formula for compound X. C=O (ii) Methanol is changed into compound X by dehydrogenation. Suggest what is meant by dehydrogenation. (b) In carbonylation, methanol reacts with compound Y forming H (ii) Loss of hydrogen only ethanoic acid. Standard Grade Chemistry Suggest a name for compound Y. (b) Carbon monoxide Unit 15 Revision. Q. The flow diagram shows what happens to starchy foods after they have been eaten. ((a) What type of substance, present in the digestive system, speeds up the breakdown of starchy foods? (b) What type of chemical reaction takes place when starch is broken down into glucose during digestion. (c) Process Y provides the body with energy. Name this process. Standard Grade Chemistry (d) Name an isomer of glucose. (a) Enzyme (d) Fructose (b) Hydrolysis (c) Respiration Unit 15 Revision. Q Ailsa carried out the experiment shown below. (a) What type of chemical reaction takes place when starch is heated with hydrochloric acid? (a) Hydrolysis (b) Fructose or maltose. (b) Ailsa said that the starch had turned into glucose. Name another sugar which turns Benedict’s solution red/orange. (c) Acid has (c) Ailsa repeated her experiment using amylase solution instead of hydrochloric acid. Suggest a reason why the Benedict’s solution did not tune red/orange. Standard Grade Chemistry denatured the enzyme








